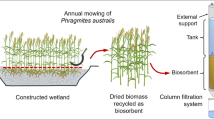
Abstract
Heavy metals are causing serious environmental and health problems worldwide, especially in places where mining is one of the major drivers of the country’s economy. Conventional technologies are considered expensive when providing the safe water; for this reason, new clean water technologies are needed. Biosorption has gained attention as a cost-effective system that uses biological materials to remove heavy metals from water; however, it can be noted that an efficient and proven biosorbent for several heavy metal has not been found. Reed (Phragmites australis) has demonstrated to be a potential biosorbent to remove several heavy metals because it is commonly found as heavy metal accumulator in wetlands. This study is focused on mercury (Hg) removal by using reed as biosorbent. Batch experiments were conducted and the microstructure of the biosorbent was analyzed by scanning electron microscope. The Langmuir isotherm and Freundlich isotherm model was applied for the data obtained. The pseudo-first-order and pseudo-second-order models were used to test adsorption kinetics data to investigate the mechanism of biosorption. A comparison with the performance of various adsorbents reported in literature was made. The results contribute to understand the use of Phragmites australis as potential biomass for biosorbent technology since it removed mercury (Hg) effectively in high concentrations. This study supports a variety of researches to achieve clean water technologies, and biosorption has proved to be a useful alternative to the conventional systems for the removal of heavy metal ions from aqueous solution.





Similar content being viewed by others
References
Al Rmalli, S. W., Dahmani, A. A., Abuein, M. M., & Gleza, A. A. (2008). Biosorption of mercury from aqueous solutions by powdered leaves of castor tree (Ricinus communis L.). Journal of Hazardous Materials, 152, 955–959.
Alloway, B. J. (2013). Heavy metals and metalloids as micronutrients for plant and animals. In B. Alloway (Ed.), Heavy Metals in Soils. Environmental Pollution, vol 22. Dordrecht: Springer.
Amabilis-Sosa LE, Siebe C, Moeller-Chávez G, Duran-Domínguez-De-Bazúa MC (2016) Remoción de mercurio por Phragmites australis empleada como barrera biológica en humedales artificiales inoculados con cepas tolerantes a metales pesados. Revista Internacional de Contaminación Ambiental 32(1):47–53. https://www.revistascca.unam.mx/rica/index.php/rica/article/view/45493.
Ashraf, M. A., Mahmood, K., Wajid, A., Maah, M. J. & Yusoff, I. 2011. Study of low cost biosorbent for biosorption of heavy metals. International conference on food engineering and biotechnology, 9, 60–68.
Bulgariu, L., Ratoi, M., Bulgariu, D., & Macoveanu, M. (2009). Adsorption potential of mercury (II) from aqueous solution onto Romanian peat moss. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 44(7), 700–706.
Clarkson, T. W., & Magos, L. (2006). The toxicology of mercury and its chemical compounds. Critical Review in Toxicology, 36, 609–662.
Coelho, J. P., Pereira, M. E., Duarte, A., & Pardal, M. A. (2005). Macroalgae response to a mercury contamination gradient in a temperate coastal lagoon (Ria de Aveiro, Portugal). Estuarine, Coastal and Shelf Science, 65, 492–500.
Deans, J. R., & Dixon, B. G. (1998). Uptake of Pb2+ and Cu2+ by novel biopolymers. Water Research, 26, 469–472.
Duffus, J. H. (2002). “Heavy metals”—a meaningless term? Pure and Applied Chemistry, 74(5), 793–807.
Duruibe, J. O., Ogwuegbu, M. O. C., & Egwurugwu, J. N. (2007). Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences, 2, 112–118.
Eom, Y., Won, J. H., Ryu, J. Y., & Lee, T. G. (2011). Biosorption of mercury (II) ions from aqueous solution by garlic (Allium sativum L.) powder. Korean Journal of Chemical Engineering, 28(6), 1439–1443.
Farooq, U., Kozinski, J. A., Khan, M. A., & Athar, M. (2010). Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresource Technology, 101, 5043–5053.
Febrianto, J., Kosasih, A. N., Sunarso, J., Ju, Y. H., Indraswati, N., & Ismadji, S. (2009). Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. Journal Hazardous Materials, 162(2–3), 616–645.
Gupta, V. K., Gupta, M., & Sharma, S. (2001). Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminum industry waste. Water Research, 35(5), 1125–1134.
Hashim, M. A., Mukhopadhyay, S., Sahu, J. N., & Sengupta, B. (2011). Remediation technologies for heavy metal contaminated ground water. Journal of Environmental Management, 92, 2355–2388.
Herrero, R., Lodeiro, P., Rey-Castro, C., Vilariño, T., & Sastre de Vicente, M. E. (2005). Removal of inorganic mercury from aqueous solutions by biomass of the marine macrolaga Cystoseira baccata. Water Research, 39, 3199–3210.
Ho, Y. S. (2006). Second-order kinetic model for sorption of cadmium onto tree fern: a comparison of linear and non–linear methods. Water Research, 40(1), 119–125.
Ho, Y. S., & McKay, G. (1999). The sorption of lead (II) ions on peat. Water Research, 33(2), 578–584.
Ho, Y. S., Wase, D., & Forster, C. (1996). Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA, 22, 219–224.
Khoramzadeh, E., Nasernejad, B., & Halladj, R. (2013). Mercury biosorption from aqueous solutions by sugarcane bagasse. Journal of the Taiwan Institute of Chemical Engineers, 44, 266–269.
Langmuir, I. (1917). The constitution and fundamental properties of solids and liquids. Journal of the American Chemical Society, 39, 1848.
Largitte, A., & Pasquier, R. (2016). A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbón. Chemical Engineering Research and Design, 109, 495–504.
Lominchar, M. A., Sierra, M. J., & Millan, R. (2015). Accumulation of mercury in Typha domingensis under field conditions. Chemosphere, 119, 994–999.
Marrugo-Negrete, J., Enamorado-Montes, G., Durango-Hernandez, J., Pinedo-Hernandez, J., & Dies, S. (2017). Removal of mercury from gold mines effluents using Limmoncharis flava in constructed wetlands. Chemosphere, 167, 188–192.
McKay, G., & Ho, Y. S. (1999). Pseudo–second order model for sorption processes. Process Biochemistry, 34, 451–465.
Montazer-Rahmatia, M. M., Rabbania, R., Abdolalia, A., & Keshtkarb, A. R. (2011). Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. Journal of Hazardous Materials, 185, 401–407.
Morel, F. M. M., Kraepiel, A. M. L., & Amyot, M. (1998). The chemical cycle and bioaccumulation of mercury. Annual Review of Ecology, Evolution, and Systematics, 29, 543–566.
Müller, A. K., Westergaard, K., Christensen, S., & Sørensen, S. J. (2001). The effect of long–term mercury pollution on the soil microbial community. FEMS Microbiology Ecology, 36(1), 11–19.
Murata, K. & Sakamoto, M. (2011). Minamata Disease. In J. O. Nriagu (Ed.), Encyclopidia of Environmental Health, volume 3, pp. 774–780, Elsevier.
Plaza, J., Viera, M., Donati, E., & Guibal, E. (2011). Biosorption of mercury by Macrocystis pyrifera and Undaria pinnatifida: influence of zinc, cadmium and nickel. Journal of Environmental Sciences, 23(11), 1778–1786.
Rae, I. B., Gibb, S. W., & Lu, S. (2009). Biosorption of Hg from aqueous solutions by crab carapace. Journal of Hazardous Materials, 164, 1601–1604.
Rawajfih, Z., & Nsour, N. (2008). Thermodinamic analysis of sorption isotherms of chromium (VI) anionic species on reed biomass. The Journal of Chemical Thermodynamics, 40, 846–851.
Sag, Y., & Kutsal, T. (2001). Recent trends in the biosorption of heavy metals: a review. Biotechnology and Bioprocess Engineering, 6(6), 376–385.
Saglam, A., Yalcinkaya, Y., Denizli, A., Arica, M. Y., Genc, Ö., & Bektas, S. (2002). Biosorption of mercury by carboxymethylcellulose and immobilized Phanerochaete chrysosporium. Microchemical Journal, 71, 73–81.
Sanita di Toppi, L., & Gabbrielli, R. (1999). Response to cadmium in higher plants. Environmental and Experimental Botany, 41, 105–130.
Sari, A., & Tuzen, M. (2008). Biosorption of cadmium (II) from aqueous solution by red algae, (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials, 157, 448–454.
Soto-Rios, P. C., Nakano, K., Fujibayashi, M., Leon-Romero, M., & Nishimura, O. (2014). Lead removal efficiency using biosorbents as alternative materials for permeable reactive barriers. Water Science and Technology, 70(2), 307–314.
Soto-Rios, P. C., Nakano, K., Leon-Romero, M., Aikawa, Y., Arai, S., & Nishimura, O. (2015). Differences in the removal mechanism of Undaria pinnatifida and Phragmites australis as biomaterials for lead removal. Water Science and Technology, 72(7), 1226–1233.
Southichak, B., Nakano, K., Nomura, M., Chiba, N., & Nishimura, O. (2006). Phragmites australis: a novel biosorbent for the removal of heavy metals from aqueous solution. Water Research, 40, 2295–2302.
Srivastava, J., Kalra, S. J. S., & Naraian, R. (2014). Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Applied Water Science, 4, 193–202.
Srivastava, S., Agrawal, S. B., & Mondal, M. K. (2015). Biosorption isotherms and kinetics on removal of Cr (VI) using native and chemically modified Lagerstroemia speciosa bark. Ecological Engineering, 85, 56–66.
Srivastavaa, P., Singha, B., & Angoveb, M. (2005). Competitive adsorption behavior of heavy metals on kaolinite. Journal of Colloid and Interface Science, 290(1), 28–38.
Svecova, L., Spanelova, M., Kubal, M., & Guibal, E. (2006). Cadmium, lead and mercury biosorption on waste fungal biomass issued from fermentation industry. I, equilibrium studies. Separation and Purification Technology, 52, 142–153.
Tan, K. F., Chu, K. H., & Hashim, M. A. (2002). Continuous packed bed biosorption of copper by immobilized seaweed biomass. European Journal of Mineral Processing and Environmental Protection, 2(3), 246–252.
U.S. Environmental Protection Agency. (1985). Drinking water criteria document for mercury. Final draft. Cincinnati: Environmental criteria and assessment office.
Velasquez, L., & Dussan, J. (2009). Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. Journal of Hazardous Materials, 167, 713–716.
Yabanli, M., Yozukmaz, A., & Sel, F. (2014). Heavy metal accumulateon in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): an example of Kadlin Creek (Mugla, Turkey). Brazilian Archives of Biology and Technology, 57(3), 434–440.
Yavuz, H., Denizli, A., Güngünes, H., Safarikova, M., & Safarik, I. (2006). Biosorption of mercury on magnetically modified yeast cells. Separation and Purification Technology, 52, 253–260.
Zeroual, Y., Moutaouakkil, A., Dzairi, F. Z., Talbi, M., Chung, P. U., Lee, K., & Blaghen, M. (2003). Biosorption of mercury from aqueous solution by Ulva lactuca biomass. Bioresource Technology, 90, 349–351.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto-Ríos, P.C., León-Romero, M.A., Sukhbaatar, O. et al. Biosorption of Mercury by Reed (Phragmites australis) as a Potential Clean Water Technology. Water Air Soil Pollut 229, 328 (2018). https://doi.org/10.1007/s11270-018-3978-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3978-8