Abstract
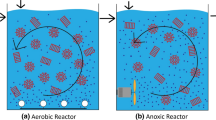
An airlift biofilm reactor was employed to study phenol biodegradation by Pseudomonas putida. Hydrodynamic tests were also conducted in a conventional column to facilitate the comparison of the dynamic behavior in different types of columns. The three-phase airlift column offered better aeration than the conventional column as liquid and solid circulation in the downcomer favored bubble breakup, increasing oxygen dissolved in the liquid phase and favoring the phenol biodegradation process. Kinetic parameters of phenol biodegradation by P. putida were obtained in an agitated batch reactor, with the initial phenol concentration varying from 10 to 750 mg/L. Experimental data were fitted using different microbial growth models found in literature. The Yano and Koga model, which considers the formation of multiple inactive enzyme–substrate complexes, fitted well with our experimental data, with a correlation coefficient, R 2 = 0.952. An internal loop airlift bioreactor was used for aerobic phenol biodegradation in which polystyrene particles were utilized to support biomass immobilization. Several tests were performed by varying the influent phenol concentration, hydraulic retention time, upstream flow, and superficial air velocity. It was concluded that until an influent phenol concentration of approximately 300 mg/L, phenol acted as the limiting substrate. For higher phenol concentrations, oxygen became the limiting substrate. An increase in the oxygen concentration resulted in the complete consumption of phenol under high phenol concentration of 500 mg/L.














Similar content being viewed by others
Abbreviations
- A :
-
cross-sectional area (m2)
- C * :
-
saturated concentration of dissolved oxygen (mg O2/L)
- C DO :
-
dissolved oxygen concentration (mg O2/L)
- C l :
-
bulk concentration of dissolved oxygen (mg O2/L)
- C 0 :
-
initial concentration of oxygen (mg O2/L)
- C phenol :
-
phenol concentration (mg/L)
- C O2 dissolved :
-
dissolved oxygen concentration (mg O2/L)
- D C :
-
column diameter (m)
- g :
-
gravity (m/s2).
- H :
-
effective bed height (m)
- H C :
-
column height (m)
- H LA :
-
liquid level (m)
- K i :
-
substrate inhibition constant (–)
- K La:
-
volumetric gas–liquid mass transfer coefficient (s−1)
- K s :
-
half saturation constant (mg/L)
- K 1, K 2 :
-
substrate inhibition models constants (mg/L)
- M s :
-
mass of solid particles (kg)
- m, n :
-
substrate inhibition models constants (–)
- R 2 :
-
correlation coefficient (–)
- S :
-
substrate concentration (mg/L)
- S m :
-
substrate concentration correspondent to the maximum specific growth rate (mg/L)
- S * :
-
substrate inhibition models constant (mg/L)
- t :
-
time (h)
- t g :
-
generation time (h)
- μ g :
-
superficial gas velocity (cm/s).
- μ g * :
-
dimensionless gas velocity (–).
- u l :
-
superficial liquid velocity (cm/s)
- X :
-
dry cell concentration (g/L)
- ε :
-
bed porosity (–)
- ε g :
-
gas holdup (–)
- ε g * :
-
relative gas holdup (–)
- ε l :
-
liquid holdup (–)
- μ :
-
specific growth rate of cells (h−1)
- μ max :
-
maximum specific growth rate (h−1)
- ρ g :
-
density of the gas phase (kg/m3)
- ρ l :
-
density of the liquid phase (kg/m3)
- ρ s :
-
density of the solid particles (kg/m3)
- COD:
-
chemical oxygen demand
- DO:
-
dissolved oxygen
- HRT:
-
hydraulic retention time
- OD:
-
optical density
- RMSE:
-
root-mean-square error
References
Aiba, S., Shoda, M., & Nagatami, M. (1968). Kinetics of product inhibition in alcohol fermentation. Biotechnology and Bioengineering, 10, 845–864.
Amorim, E. L. C., Sader, L. T., & Silva, E. L. (2015). Effects of the organic-loading rate on the performance of an anaerobic fluidized-bed reactor treating synthetic wastewater containing phenol. Journal of Environmental Engineering, 141(10), 04015022-1–04015022-9.
APHA. (1998). Standard methods for the examination for water and wastewater (20th ed.). Washington, DC: American Public Health Association/American Water Works Association/Water Environmental Federation.
Basak, B., Bhunia, B., Dutta, S., Chakraborty, S., & Dey, A. (2014). Kinetics of phenol biodegradation at high concentration by a metabolically versatile isolated yeast Candida tropicalis PHB5. Environmental Science and Pollution Research, 21, 1444–1454.
Begum, S. S., & Radha, K. V. (2013). Biodegradation kinetic studies on phenol in internal draft tube (inverse fluidized bed) biofilm reactor using Pseudomonas fluorescens: performance evaluation of biofilm and biomass characteristics. Bioremediation Journal, 17(4), 264–277.
Begum, S. S., & Radha, K. V. (2014). Hydrodynamic behavior of inverse fluidized bed biofilm reactor for phenol biodegradation using Pseudomonas fluorescens. Korean Journal of Chemical Engineering, 31(3), 436–445.
Borighem, G., & Vereecken, J. (1981). Model of a chemostat utilizing phenol as inhibitory substrate. Ecological Modelling, 12, 231–243.
Briggs, G. E., & Haldane, J. B. (1925). A note on the kinetics of enzyme action. The Biochemical Journal, 19(2), 338–339.
Chung, T.-P., Tseng, H.-Y., & Juang, R.-S. (2003). Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochemistry, 38, 1497–1507.
Combarros, R. G., Rosas, I., Lavín, A. G., Rendueles, M., & Díaz, M. (2014). Influence of biofilm on activated carbon on the adsorption and biodegradation of salicylic acid in wastewater. Water, Air, & Soil Pollution, 225, 1858–1869.
CONAMA’s Resolution N° 430 (2011). “Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução no 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente-CONAMA.”
Dapaals, S. Y., & Hill, G. A. (1992). Biodegradation of chlorophenol mixtures by Pseudomonas putida. Biotechnology and Bioengineering, 40, 1353–1358.
Dash, R. R., Gaur, A., & Balomajumder, C. (2009). Cyanide in industrial wastewaters and its removal: a review on biotreatment. Journal of Hazardous Materials, 163, 1–11.
Deckwer, W.-D., Louisi, Y., Zaidi, A., & Ralek, M. (1980). Hydrodynamic properties of the Fisher-Tropsch slurry process. Industrial & Engineering Chemistry Process Design and Development, 19(4), 699–708.
Dey, S., & Mukherjee, S. (2010). Kinetic studies for an aerobic packed bed biofilm reactor for treatment of organic wastewater with and without phenol. Journal of Water Resource and Protection, 2, 731–738.
Edwards, V. H. (1970). The influence of high substrate concentrations on microbial kinetics. Biotechnology and Bioengineering, 7, 679–712.
González, G., Herrera, M. G., García, M. T., & Peña, M. M. (2001). Biodegradation of phenol in a continuous process: comparative study of stirred tank and fluidized-bed bioreactors. Bioresource Technology, 76, 245–251.
Han, K., & Levenspiel, O. (1988). Extended Monod kinetics for substrate, product, and cell inhibition. Biotechnology and Bioengineering, 32, 430–437.
Heijnen, J. J., Hols, J., van der Lans, R. G. J. M., van Leeuwen, H. L. J. M., Mulder, A., & Weltevrede, R. (1997). A simple hydrodynamic model for the liquid circulation velocity in a full-scale two- and three-phase internal airlift reactor operating in the gas recirculation regime. Chemical Engineering Science, 52(15), 2527–2540.
Hirata, A., Hosaka, Y., & Umezawa, H. (1990). Characteristics of simultaneous utilization of oxygen and substrate in a three-phase fluidized bed bioreactor. Journal of Chemical Engineering of Japan, 23(3), 303–307.
Ismail, Z. Z., & Khudhair, H. A. (2015). Recycling of immobilized cells for aerobic biodegradation of phenol in a fluidized bed bioreactor. Systemics, Cybernetics and Informatics, 13(5), 81–86.
Jalilnejad, E., & Vahabzadeh, F. (2014). Use of packed-bed airlift reactor with net draft tube to study kinetics of naphthalene degradation by Ralstonia eutropha. Environmental Science and Pollution Research, 21, 4592–4604.
Kantarci, N., Borak, F., & Ulgen, K. O. (2005). Bubble column reactors. Process Biochemistry, 40, 2263–2283.
Karamanev, D. G., Nagamune, T., & Endo, I. (1992). Hydrodynamic and mass transfer study of a gas–liquid–solid draft tube spouted bed bioreactor. Chemical Engineering Science, 47(13/14), 3581–3588.
Kato, Y., Aki, N. A., Fukuda, T., & Tanaka, S. (1972). The behavior of suspended particles and liquid in bubble columns. Journal of Chemical Engineering of Japan, 5(2), 112–118.
Kawagoe, K., Inoue, T., Nakao, K., & Otake, T. (1976). Flow-pattern and gas holdup conditions in gas-sparged contactors. International Journal of Chemical Engineering, 16, 176–183.
Kim, S. D., Baker, C. G. J., & Bergougnou, M. A. (1975). Phase holdup characteristics of three phase fluidized beds. The Canadian Journal of Chemical Engineering, 53, 134–139.
Kishore, K. A., & Reddy, G. V. (2012). Studies on phenol biodegradation rates in waste water treatment by draft tube fluidized bed bioreactor. International Journal of Chemical Sciences and Applications, 3(2), 249–253.
Kotturi, G., Robinson, C. W., & Inniss, W. E. (1991). Phenol degradation by a psychrotrophic strain of Pseudomonas putida. Applied Microbiology and Biotechnology, 34, 539–543.
Kumar, A., Kumar, S., & Kumar, S. (2005). Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochemical Engineering Journal, 22, 151–159.
Li, H., & Prakash, A. (1997). Heat transfer and hydrodynamics in a three-phase slurry bubble column. Industrial & Engineering Chemistry Research, 36(11), 4688–4694.
Li, Y., Li, J., Wang, C., & Wang, P. (2010). Growth kinetics and phenol biodegradation of psychrotrophic Pseudomonas putida LY1. Bio/Technology, 101, 6740–6744.
Livingston, A. G., & Chase, H. A. (1989). Modeling phenol degradation in a fluidized-bed bioreactor. AICHE Journal, 35(12), 1980–1992.
Luong, J. H. T. (1987). Generalization of Monod kinetics for substrate, product, and cell inhibition. Biotechnology and Bioengineering, 32, 242–248.
Monod, J. (1949). The growth of bacterial cultures. Annual Review of Microbiology, 3(1), 371–394.
Monteiro, A. A. M. G., Boaventura, R. A. R., & Rodrigues, A. E. (2000). Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochemical Engineering Journal, 6, 45–49.
Mordocco, A., Kuek, C., & Jenkins, R. (1999). Continuous degradation of phenol at low concentration using immobilized Pseudomonas putida. Enzyme and Microbial Technology, 25, 530–536.
Ranjbar, S., Aghtaei, H. K., Jalilnejad, E., & Vahabzadeh, F. (2016). Application of an airlift reactor with a net draft tube in phenol bio-oxidation using Ralstonia eutropha. Desalinization and Water Treatment, 57, 1–13.
Rozich, A. F. & Gaudy, A. F. Jr. (1986). Process technology for the biological treatment of toxic organic wastes. Hazardous and Industrial Solid Waste Testing and Disposal: Sixth Volume. ASTM STP 933, D.
Sathya, R., Rasi, M., & Rajendran, L. (2015). Non-linear analysis of Haldane kinetic model in phenol degradation in batch operations. Kinetics and Catalysis, 56(2), 141–146.
Shah, Y. T., Kelkar, B. G., Godbole, S. P., & Deckwer, W.-D. (1982). Design parameters estimations for bubble column reactors. AICHE Journal, 28(3), 353–379.
Sedighi, M., & Vahabzadeh, F. (2014). Kinetic modeling of cometabolic degradation of ethanethiol and phenol by Ralstonia eutropha. Biotechnology and Bioprocess Engineering, 19, 239–249.
Silva, E. L. (1995). Aerobic treatment of phenol in three-phase fluidized bed reactor. Ph.D. thesis. Department of Hydraulic and Sanitation—University of São Paulo, São Carlos, Brazil (in Portuguese).
Singh, N., & Balomajumder, C. (2016). Simultaneous biosorption and bioaccumulation of phenol and cyanide using coconut shell activated carbon immobilized Pseudomonas putida (MTCC 1194). Journal of Environmental Chemical Engineering, 4, 1604–1614.
Tang, W.-T., & Fan, L.-S. (1987). Steady state phenol degradation in a draft-tube, gas–liquid–solid fluidized-bed bioreactor. AICHE Journal, 33(2), 240–249.
Tziotzios, G., Teliou, M., Kaltsouni, V., Lyberatos, G., & Vayenas, D. V. (2005). Biological phenol removal using suspended growth and packed bed reactors. Biochemical Engineering Journal, 26(1), 65–71.
Ucun, H., Yildiz, E., & Nuhoglu, A. (2010). Phenol biodegradation in a batch jet loop bioreactor (JLB): kinetics study and pH variation. Bio/Technology, 101, 2965–2971.
Vasalos, A. I., Bild, E. M., Rundell, D. N., & Tatterson, D. F. (1980). Experimental techniques for studying fluid dynamics of H-coal reactor. Coal Processing Technology, 6, 226–228.
Viggiani, A., Olivieri, G., Siani, L., Di Donato, A., Marzocchella, A., Salatino, P., Barbieri, P., & Galli, E. (2006). An airlift reactor for the biodegradation of phenol by Pseudomonas stutzeri OX1. Journal of Biotechnology, 123, 464–477.
Wang, S. J., & Loh, K. C. (1999). Modeling the role of metabolic intermediates in kinetics of phenol biodegradation. Enzyme and Microbial Technology, 25, 177–184.
Wolski, E. A., Durruty, I., Haure, P. M., & González, J. F. (2012). Penicillium chrysogenum: phenol degradation abilities and kinetic model. Water, Air, & Soil Pollution, 223, 2323–2332.
Yamane, T. (1967). Statistics—an introductory analysis. New York: Harper and Brown.
Yano, T., & Koga, S. (1969). Dynamic behaviour of the chemostat subject to substrate inhibition. Biotechnology and Bioengineering, 11, 139–153.
Ying, D. H., Givens, E. N., & Weimer, R. F. (1980). Gas holdup in gas–liquid and gas–liquid–solid flow reactors. Industrial & Engineering Chemistry Process Design and Development, 19(4), 635–638.
Acknowledgements
This work was supported by the CNPq—National Council for Scientific and Technological Development, CAPES—Coordination for the Improvement of Higher Education Personnel, and FAPESP—São Paulo Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertollo, F.B., Lopes, G.C. & Silva, E.L. Phenol Biodegradation by Pseudomonas putida in an Airlift Reactor: Assessment of Kinetic, Hydrodynamic, and Mass Transfer Parameters. Water Air Soil Pollut 228, 398 (2017). https://doi.org/10.1007/s11270-017-3569-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3569-0