Abstract
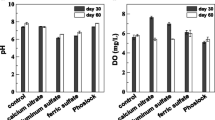
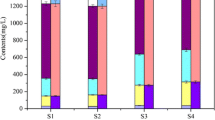
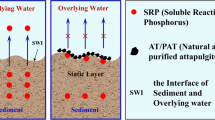
Effects of the combined application of ferrihydrite-modified diatomite (FHMD) and gypsum on phosphorus control were investigated in a laboratory-scale artificial aquarium under anoxic and agitation conditions over 120 days. Daily oscillation of a metal grid to simulate agitating effects by wind did not yield the sediment resuspension in the 120-day treatment aquarium (120-day aquarium) due to the gypsum stabilization, while significant sediment resuspension was observed in the control aquarium. The combined application of FHMD and gypsum did not affect the total kjeldahl nitrogen (TKN) concentrations in both the control aquarium and the 120-day aquarium. Under anoxic conditions and sediment resuspension conditions, a large increase in total phosphorus (TP) concentrations was observed in the control aquarium. However, the TP concentrations in the 120-day aquarium stayed relatively stable, within a range of 9.1–13.3 μg/L. After the 120 days’ incubation, translocation from mobile labile-P and organic-P to P adsorbed by FHMD occurred. The combined application of FHMD and gypsum effectively maintained TP levels within the oligotrophic range under anoxic and agitation conditions in the laboratory-scale artificial aquarium by removing phosphorus from lake water and reducing sedimentary phosphorus release via gypsum sediment stabilization and FHMD phosphorus immobilization.






Similar content being viewed by others
References
American Public Health Association (APHA), American Water Works Association and Water Environmental Federation. (2012). Standard methods for the examination of water and wastewater (22nd ed.). Washington, D.C.: American Public Association.
Bonin, D. J., & Golterman, H. L. (1989). Fluxes between trophic levels and through the water-sediment interface. Proceedings of the Joint Congress of Limnology and Oceanography. France: Marseille.
Cermelj, B., & Faganeli, J. (2003). Anoxic degradation of biogenic debris in sediments of eutrophic subalpine Lake Bled (Slovenia). Hydrobiologia, 494, 193–199.
Cooke, G., Welch, E., Peterson, S., & Newroth, P. (1986). Lake and reservoir restoration. Stoneham: Butterworth Publisher.
Cooke, G. D., Welch, E. B., Peterson, S. A., & Nichols, S. A. (2005). Restoration and management of lakes and reservoirs (3rd ed.). New York: Taylor & Francis.
de Boer, W. F. (2007). Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia, 591, 5–24.
Dokulil, M. T., & Teubner, K. (2003). Eutrophication and restoration of shallow lakes – the concept of stable equilibria revisited. Hydrobiologia, 506–509, 29–35.
Forsberg, C. (1989). Importance of sediments in understanding nutrient cyclings in lakes. Hydrobiologia, 176(177), 263–277.
Gächter, R., & Müller, B. (2003). Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol Oceanogr, 48, 929–933.
Gokie, T., & Beckman, R. (2006). The utilization of fly ash for sediment stabilization during the Holmes Lake restoration project. World Environmental and Water Resource Congress, 2006, 1–10.
Hoge, V. R., Conrow, R., Coveney, M., & Peterson, J. (2003). The application of alum residual as a phosphorus abatement tool within the Lake Apopka restoration area. WEF/AWWA/CWEA Joint Residuals and Biosolids Management, 2003, 1500–1513.
Jensen, H. S., & Thamdrup, B. (1993). Iron-bound phosphorus in marine sediments as measured by bicarbonate-dithionite extraction. Hydrobiologia, 253, 47–59.
Kalff, J. (2002). Limnology inland water ecosystems. New Jersey: Prentice Hall.
Konopka, A., & Brock, T. D. (1978). Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Applied Environmental Microbiology, 36, 572–576.
Koski-Vähälä, J., Hartikainen, H., & Tallberg, P. (2001). Phosphorus mobilization from various sediment pools in response to increased pH and silicate concentration. J Environ Qual, 30, 546–552.
Kukkadapu, R. K., Zachara, J. M., Fredrickson, J. K., Smith, S. C., Dohnalkova, A. C., & Russell, C. K. (2003). Transformation of 2-line ferrihydrite to 6-line ferrihydrite under oxic and anoxic conditions. Am Mineral, 88, 1903–1914.
Laenen, A., & LeTourneau, A. P. (1996). Estimate of wind-induce resuspension of bed sediment during periods of low lake elevation. Upper Klamath Basin nutrient loading study. Portland, Oregon: U. S. geological Survey.
McCulloch, J., Gudimov, A., Arhonditsis, G., Chesnyuk, A., & Dittrich, M. (2013). Dynamics of P-binding forms in sediments of a mesotrophic hard-water lake: insights from non-steady state reactive-transport modeling, sensitivity and identifiability analysis. Chem Geol, 354, 216–232.
Meadows, A., Meadows, P. S., West, F. J. C., & Murray, J. M. H. (2000). Bioturbation, geochemistry and geotechnics of sediments affected by the oxygen minimum zone on the Oman continental slope and abyssal plain, Arabian Sea. Deep-Sea Research Part II, 47, 259–280.
Oliver, R. L., Hamilton, D. P., Brookes, J. D., & Ganf, G. G. (2012). Physiology, blooms and prediction of planktonic cyanobacteria. In B. A. Whitton (Ed.), Ecology of cyanobacteria II, their diversity in space and time (pp. 155–194). New York: Springer Dordrecht Heidelberg.
Phlips, E. J., Cichra, M., Havens, K., Hanlon, C., Badylak, S., Rueter, B., Randall, M., & Hansen, P. (1997). Relationships between phytoplankton dynamics and the availability of light and nutrients in a shallow sub-tropical lake. J Plankton Res, 19, 319–342.
Ruloy, J. E., & Rusch, K. A. (2004). Development of a simplified phosphorus management model for a shallow, subtrophical, urban hypereutrophic lake. Ecol Eng, 22, 77–98.
Rydin, E. (2000). Potentially mobile phosphorus in lake Erken sediment. Water Res, 34, 2037–2042.
Salonen, V.-P., & Varjo, E. (2000). Gypsum treatment as a restoration method for sediments of eutrophic lakes experiments from southern Finland. Environ Geol, 39, 353–359.
Schenau, S. J., Slomp, C. P., & De Lange, G. J. (2000). Phosphogenesis and active phosphorite formation in sediments from the Arabian Sea oxygen minimum zone. Mar Geol, 169, 1–20.
Sutula, M., Bianchi, T. S., & McKee, B. A. (2004). Effect of seasonal sediment storage in the lower Mississippi River on the flux of reactive particulate phosphorus to the Gulf of Mexico. Limnol Oceanogr, 49, 2223–2235.
Wetzel, R. G. (2001). Limnology lake and reservoir ecosystems (3rd ed.). London: Academic.
Xiong, W. (2009). Development and application of ferrihydrite-modified diatomite and gypsum for phosphorus control study in lakes and reservoirs. Ph.D. dissertation: University of Saskatchewan, Saskatoon, Canada.
Xiong, W., & Peng, J. (2008). Development and characterization of ferrihydrite-modified diatomite as a phosphorus adsorbent. Water Res, 42, 4869–4877.
Xiong, W., & Peng, J. (2011). Laboratory-scale investigation of ferrihydrite-modified diatomite as a phosphorus co-precipitant. Water Air Soil Pollut, 215, 645–654.
Xiong, W., Peng, J., & Hu, Y. (2012). Use of X-ray absorption near edge structure (XANES) to identify physisorption and chemisorption of phosphate onto ferrihydrite-modified diatomite. J Colloid Interface Sci, 368, 528–532.
Acknowledgments
The research project was supported through an NSERC discovery research grant from the Natural Sciences and Engineering Research Council of Canada and an international research grant from China. The authors are grateful to Mr. D. Fisher, technologist at the University of Saskatchewan, for his assistance in the environmental laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, W., Peng, J. Combined Application of Ferrihydrite-Modified Diatomite and Gypsum to Phosphorus Control in a Laboratory-Scale Artificial Aquarium. Water Air Soil Pollut 225, 1855 (2014). https://doi.org/10.1007/s11270-013-1855-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1855-z