Abstract
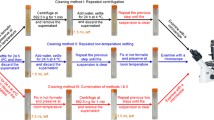
Cryptosporidium parvum oocysts may reach soil through direct deposition of human or animal fecal material, irrigation with raw wastewater or untreated effluents, and contaminated runoff. Examination of soil samples for oocyst presence is of primary importance in order to prevent secondary contamination of crops and groundwater. Several methods were proposed for oocyst recovery from soil samples; however, their efficiency was very low. In the present study, four known methods used to recover oocysts from water and fecal samples (sedimentation, sedimentation with reduced water content, sucrose floatation, water–ether separation) were compared to a method used in the past to recover bacterial spores from bottom sediments (two-phase separation). The two-phase separation technique proved to be the best method of choice resulting in a recovery average of 61.2 ± 15.6%. According to this method, the lowest and highest recoveries were 37% to 95%, respectively. Two other important outcomes were observed with the soil experimental set-up: (1) recovery efficiency is influenced by oocyst viability (high viability was directly correlated with increased recovery efficiency) and (2) high sand content of soil samples reduced oocyst recovery by its detrimental effect on oocyst viability.


Similar content being viewed by others
References
Armon, R., Gold, D., Brodsky, M., & Oron, G. (2002). Surface and subsurface irrigation with effluents of different qualities and presence of Cryptosporidium oocysts in soil and on crops. Water Science and Technology, 46(3), 115–122.
Barwick, R. S., Mohammed, H. O., White, A. B., & Bryan, R. T. (2000). Detection of Cryptosporidium parvum and Cryptosporidium muris in soil samples. Biology and Fertility of Soils, 31(5), 385–390. doi:10.1007/s003749900185.
Boyer, D. G., & Kuczynska, E. (2003). Storm and seasonal distributions of fecal coliforms and Cryptosporidium in a spring. Journal of the American Water Resources Association, 39(6), 1449–1456. doi:10.1111/j.1752-1688.2003.tb04430.x.
Bukhari, Z., & Smith, H. V. (1995). Effect of three concentration techniques on viability of Cryptosporidium parvum oocysts recovered from bovine feces. Journal of Clinical Microbiology, 33(10), 2592–2595.
Chacin-Bonilla, L., Barrios, F., & Sanchez, Y. (2008). Environmental risk factors for Cryptosporidium infection in an island from Western Venezuela. Memorias do Instituto Oswaldo Cruz, 103(1), 45–49. doi:10.1590/S0074-02762008005000007.
Chesnot, T., & Schwartzbrod, J. (2004). Quantitative and qualitative comparison of density-based purification methods for detection of Cryptosporidium oocysts in turbid environmental matrices. Journal of Microbiological Methods, 58(3), 375–386. doi:10.1016/j.mimet.2004.05.001.
Dai, X., & Boll, J. (2003). Evaluation of attachment of Cryptosporidium parvum and Giardia lamblia to soil particles. Journal of Environmental Quality, 32(1), 296–304.
Darnault, C. J. G., Garnier, P., Kim, Y.-J., Oveson, K. L., Steenhuis, T. S., Parlange, J.-Y., et al. (2003). Preferential transport of Cryptosporidium parvum oocysts in variably saturated subsurface environments. Water Environment Research, 75(2), 113–120. doi:10.2175/106143003X140890.
Duke, L. A., Breathnach, A. S., Jenkins, D. R., Harkis, B. A., & Codd, A. W. (1996). A mixed outbreak of Cryptosporidium and Campylobacter infection associated with a private water supply. Epidemiology and Infection, 116(3), 303–308.
Emerson, D. J., & Cabelli, V. J. (1982). Extraction of Clostridium perfringens spores from bottom sediment samples. Applied and Environmental Microbiology, 44(5), 1144–1149.
Fayer, R. (2004). Cryptosporidium: a water-borne zoonotic parasite. Veterinary Parasitology, 126(1–2), 37–56. doi:10.1016/j.vetpar.2004.09.004.
Gale, P. (2003). Using event trees to quantify pathogen levels on root crops from land application of treated sewage sludge. Journal of Applied Microbiology, 94(1), 35–47. doi:10.1046/j.1365-2672.2003.01794.x.
Grimason, A. M., Smith, H. V., Parker, J. F. W., Bukhari, Z., Campbell, A. T., & Robertson, L. J. (1994). Application of DAPI and immunofluorescence for enhanced identification of Cryptosporidium spp oocysts in water samples. Water Research, 28(3), 733–736. doi:10.1016/0043-1354(94)90154-6.
Higgins, J. A., Fayer, R., Trout, J. M., Xiao, L., Lal, A. A., Kerby, S., et al. (2001). Real-time PCR for the detection of Cryptosporidium parvum. Journal of Microbiological Methods, 47(3), 323–337. doi:10.1016/S0167-7012(01)00339-6.
Hijnen, W. A. M., Schijven, J. F., Bonne, P., Visser, A., & Medema, G. J. (2004). Elimination of viruses, bacteria and protozoan oocysts by slow sand filtration. Water Science and Technology, 50(1), 147–154.
Hutchison, M. L., Walters, L. D., Moore, T., Thomas, D. J. I., & Avery, S. M. (2005). Fate of pathogens present in livestock wastes spread onto fescue plots. Applied and Environmental Microbiology, 71(2), 691–696. doi:10.1128/AEM.71.2.691-696.2005.
Jenkins, M. B., Bowman, D. D., Fogarty, E. A., & Ghiorse, W. C. (2002). Cryptosporidium parvum oocyst inactivation in three soil types at various temperatures and water potentials. Soil Biology & Biochemistry, 34(8), 1101–1109. doi:10.1016/S0038-0717(02)00046-9.
Khashiboun, K., Zilberman, A., Shaviv, A., Starosvetsky, J., & Armon, R. (2007). The fate of Cryptosporidium parvum oocysts in reclaimed water irrigation—history and non-history soils irrigated with various effluent qualities. Water, Air, and Soil Pollution, 185(1–4), 33–41. doi:10.1007/s11270-007-9420-2.
Kuczynska, E., & Shelton, D. R. (1999). Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils. Applied and Environmental Microbiology, 65(7), 2820–2826.
Kuznar, Z. A., & Elimelech, M. (2005). Role of surface proteins in the deposition kinetics of Cryptosporidium parvum oocysts. Langmuir, 21(2), 710–716. doi:10.1021/la047963m.
Mawdsley, J. L., Brooks, A. E., & Merry, R. J. (1996). Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biology and Fertility of Soils, 21(1–2), 30–36. doi:10.1007/BF00335990.
Medema, G. J., Schets, F. M., Teunis, P. F. M., & Havelaar, A. H. (1998). Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Applied and Environmental Microbiology, 64(11), 4460–4466.
Neumann, N. F., Gyurek, L. L., Gammie, L., Finch, G. R., & Belosevic, M. (2000). Comparison of animal infectivity and nucleic acid staining for assessment of Cryptosporidium parvum viability in water. Applied and Environmental Microbiology, 66(1), 406–412.
Parker, J. F. W., & Smith, H. V. (1993). Destruction of oocysts of Cryptosporidium parvum by sand and chlorine. Water Research, 27(4), 729–731. doi:.1016/0043-1354(93)90184-J.
Quy, R. J., Cowan, D. P., Haynes, P. J., Sturdee, A. P., Chalmers, R. M., Bodley-Tickell, A. T., et al. (1999). The Norway rat as a reservoir host of Cryptosporidium parvum. Journal of Wildlife Diseases, 35(4), 660–670.
Ramirez, N. E., & Sreevatsan, S. (2006). Development of a sensitive detection system for Cryptosporidium in environmental samples. Veterinary Parasitology, 136(3–4), 201–213. doi:10.1016/j.vetpar.2005.11.023.
Robertson, L. J., Campbell, A. T., & Smith, H. V. (1992). Survival of Cryptosporidium parvum oocysts under various environmental pressures. Applied and Environmental Microbiology, 58(11), 3494–3500.
Robertson, L. J., Campbell, A. T., & Smith, H. V. (1998). Viability of Cryptosporidium parvum oocysts: assessment by the dye permeability assay. Applied and Environmental Microbiology, 64(9), 3544–3545.
Searcy, K. E., Packman, A. I., Atwill, E. R., & Harter, T. (2005). Association of Cryptosporidium parvum with suspended particles: impact on oocyst sedimentation. Applied and Environmental Microbiology, 71(2), 1072–1078. doi:10.1128/AEM.71.2.1072-1078.2005.
Slifko, T. R., Smith, H. V., & Rose, J. B. (2000). Emerging parasite zoonoses associated with water and food. International Journal for Parasitology, 30(12–13), 1379–1393. doi:10.1016/S0020-7519(00)00128-4.
Smith, H. V., & Rose, J. B. (1998). Waterborne cryptosporidiosis: current status. Parasitology Today (Personal Ed.), 14(1), 14–22. doi:10.1016/S0169-4758(97)01150-2.
Teunis, P. F. M., Medema, G. J., Kruidenier, L., & Havelaar, A. H. (1997). Assessment of the risk of infection by Cryptosporidium or Giardia in drinking water from a surface water source. Water Research, 31(6), 1333–1346. doi:10.1016/S0043-1354(96)00387-9.
Thurston-Enriquez, J. A., Watt, P., Dowd, S. E., Enriquez, R., Pepper, I. L., & Gerba, C. P. (2002). Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. Journal of Food Protection, 65(2), 378–382.
Walker, M., & Redelman, D. (2004). Detection of Cryptosporidium parvum in soil extracts. Applied and Environmental Microbiology, 70(3), 1827–1829. doi:10.1128/AEM.70.3.1827-1829.2004.
Walker, M. J., Montemagno, C., Bryant, J. C., & Ghiorse, W. C. (1998). Method detection limits of PCR and immunofluorescence assay for Cryptosporidium parvum in soil. Applied and Environmental Microbiology, 64(6), 2281–2283.
Wilson, J., & Margolin, A. B. (2003). Efficacy of glutaraldehyde disinfectant against Cryptosporidium parvum in the presence of various organic soils. Journal of AOAC International, 86(1), 96–100.
Zuckerman, U., Gold, D., Shelef, G., Yuditsky, A., & Armon, R.(1997) Microbial degradation of Cryptosporidium parvum by Serratia marcescens with high chitinolytic activity. In C. R. Fricker, J. L. Clancy, & P. A. Rochelle (Eds.) 1997 International Symposium on Waterborne Cryptosporidium Proceedings (pp. 297–304). AWWA, Newport Beach, California, March 2–5, 1997.
Acknowledgement
The present study was partially funded by a grant from Grand Water Research Institute (GWRI), at Technion, Haifa, Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zilberman, A., Zimmels, Y., Starosvetsky, J. et al. A Two-Phase Separation Method for Recovery of Cryptosporidium Oocysts from Soil Samples. Water Air Soil Pollut 203, 325–334 (2009). https://doi.org/10.1007/s11270-009-0015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0015-y