Abstract
Background
It is unknown whether five-year overall survival (OS) differs and to what extent between testicular germ-cell tumor (TGCT) patients and age-matched male population-based controls.
Materials
We identified newly diagnosed (2004–2014) TGCT patients within Surveillance Epidemiology and End Results database 2004–2019. We compared OS between non-seminoma (NS-TGCT) and seminoma (S-TGCT) patients relative to age-matched male population-based controls based on Social Security Administration Life-Tables. Smoothed cumulative incidence plots displayed cancer-specific mortality (CSM) vs. other-cause mortality (OCM).
Results
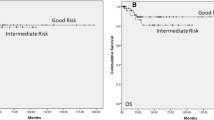
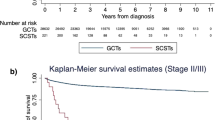
Of all 20,935 TGCT patients, 43% had NS-TGCT and 57% had S-TGCT. Of NS-TGCT patients, 63% were stage I vs. 16% stage II vs. 21% stage III. Of S-TGCT patients, 86% were stage I vs. 8% were stage II vs. 6% stage III. Five-year OS differences between NS-TGCT patients vs age-matched male population-based controls were 97 vs. 99% (Δ = 2%) for stage I, 96 vs. 99% (Δ = 3%) for stage II, 76 vs 98% (Δ = 22%) for stage III. Five-year OS differences between S-TGCT patients vs age-matched male population-based controls were 97 vs. 98% (Δ = 1%) for stage I, 95 vs. 97% (Δ = 2%) for stage II, 87 vs. 98% (Δ = 11%) for stage III. OCM rates ranged from 1 to 3% in NS-TGCT patients and from 2 to 4% in S-TGCT patients.
Conclusion
The OS difference between NS-TGCT patients vs. age-matched male population-based controls was invariably higher across all stages (2–22%) than for S-TGCT patients (1–11%). Reassuringly, OCM rates were marginal in stage I and stage II patients. Conversely, higher OCM rates were recorded in stage III patients.




Similar content being viewed by others
Data availability
All data generated for this analysis were from the SEER database (https://seer.cancer.gov/data/). The code for the analyses will be made available upon request.
References
Stephenson A, Eggener SE, Bass EB et al (2019) Diagnosis and treatment of early stage testicular cancer guideline: AUA GUIDELINE
Moch H, Cubilla AL, Humphrey PA et al (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol 70:93–105. https://doi.org/10.1016/j.eururo.2016.02.029
Cheng L, Albers P, Berney DM et al (2018) Testicular cancer. Nat Rev Dis Primer 4:29. https://doi.org/10.1038/s41572-018-0029-0
Chovanec M, Cheng L (2022) Advances in diagnosis and treatment of testicular cancer. BMJ. https://doi.org/10.1136/bmj-2022-070499
Gillessen S, Sauvé N, Collette L et al (2021) Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): Results from the IGCCCG Update Consortium. J Clin Oncol 39:1563–1574. https://doi.org/10.1200/JCO.20.03296
Beyer J, Collette L, Sauvé N et al (2021) Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-Update Consortium. J Clin Oncol 39:1553–1562. https://doi.org/10.1200/JCO.20.03292
Park JS, Kim J, Elghiaty A, Ham WS (2018) Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 97:e12390. https://doi.org/10.1097/MD.0000000000012390
Social Security Administration-Actuarial Life Table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 13 Jan 2023
SEER Cancer Statistics Review, 1975-2018. In: SEER. https://seer.cancer.gov/csr/1975_2018/index.html. Accessed 14 Jan 2023
Preisser F, Bandini M, Mazzone E et al (2019) Validation of the social security administration life tables (2004–2014) in localized prostate cancer patients within the surveillance, epidemiology, and end results database. Eur Urol Focus 5:807–814. https://doi.org/10.1016/j.euf.2018.05.006
Chierigo F, Borghesi M, Würnschimmel C et al (2022) Life expectancy in metastatic urothelial bladder cancer patients according to race/ethnicity. Int Urol Nephrol 54:1521–1527. https://doi.org/10.1007/s11255-022-03221-5
Würnschimmel C, Nocera L, Wenzel M et al (2022) Race/ethnicity may be an important predictor of life expectancy in localized prostate cancer patients: novel analyses using social security administration life tables. J Racial Ethn Health Disparities. https://doi.org/10.1007/s40615-022-01257-y
Würnschimmel C, Collà Ruvolo C, Nocera L et al (2022) Race/ethnicity determines life expectancy in surgically treated T1aN0M0 renal cell carcinoma patients. Eur Urol Focus 8:191–199. https://doi.org/10.1016/j.euf.2021.02.004
Cano Garcia C, Piccinelli ML, Tappero S et al (2023) Differences in overall survival of T2N0M0 bladder cancer patients vs. population-based controls according to treatment modalities. Int Urol Nephrol 55:1117–1123. https://doi.org/10.1007/s11255-023-03517-0
Garcia CC, Nimer N, Piccinelli ML et al (2023) Differences in overall survival between clear cell metastatic renal cell carcinoma patients versus population-based controls according to race/ethnicity in the United States. Ann Epidemiol. https://doi.org/10.1016/j.annepidem.2023.01.003
R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 14 Jan 2023
Palumbo C, Mistretta FA, Mazzone E et al (2019) Contemporary incidence and mortality rates in patients with testicular germ cell tumors. Clin Genitourin Cancer 17:e1026–e1035. https://doi.org/10.1016/j.clgc.2019.06.003
Mao W, Wu J, Kong Q et al (2020) Development and validation of prognostic nomogram for germ cell testicular cancer patients. Aging 12:22095–22111. https://doi.org/10.18632/aging.104063
Woldu SL, Moore JA, Ci B et al (2018) Practice patterns and impact of postchemotherapy retroperitoneal lymph node dissection on testicular cancer outcomes. Eur Urol Oncol 1:242–251. https://doi.org/10.1016/j.euo.2018.04.005
Noureldin YA, Alqirnas MQ, Aljarallah MF et al (2022) Testicular cancer among Saudi adults: hands on a nationwide cancer registry over 10 years. Arab J Urol 20:182–188. https://doi.org/10.1080/2090598X.2022.2084902
Yamashita S, Koyama J, Goto T et al (2020) Trends in age and histology of testicular cancer from 1980–2019: a single-center study. Tohoku J Exp Med 252:219–224. https://doi.org/10.1620/tjem.252.219
Facchini G, Rossetti S, Berretta M et al (2019) Prognostic and predictive factors in testicular cancer. Eur Rev Med Pharmacol Sci 23:3885–3891
Cano Garcia C, Panunzio A, Tappero S et al (2023) Survival of testicular pure embryonal carcinoma vs. mixed germ cell tumor patients across all stages. Medicina (Mex) 59:451. https://doi.org/10.3390/medicina59030451
Cano Garcia C, Barletta F, Incesu R-B et al (2023) Survival of testicular pure teratoma vs. mixed germ cell tumor patients in primary tumor specimens across all stages. Cancers 15:694. https://doi.org/10.3390/cancers15030694
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: SM, MLP; Acquisition of the data: SM, CCG, AB; Formal analysis: SM, ZT; Investigation and Data Curation: SM, ST, FB; Writing- Original Draft: SM, MLP, RBI, LS; Visualization: FS, VM, GC, CCR, SS, OdC, GM, FKHC, CT, AB, DT, SA, LC; Funding acquisition: N/A; Supervision: NL, PIK; Project administration: PIK.
Corresponding author
Ethics declarations
Conflict of interest
Shahrokh F. Shariat: Horonraria: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen. Consulting or Advisory Role: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen. Speakers’ Bureau: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen, Movember Foundation. Patents: Method to determine prognosis after therapy for prostate cancer - granted 2002-09-06, Methods to determine prognosis after therapy for bladder cancer - granted 2003-06-19, Prognostic methods for patients with prostatic disease - granted 2004-08-05; Soluble Fas urinary marker for the detection of bladder transitional cell carcinoma - granted 2010-07-20. Our research was conducted without any other potential conflicts of interest.
Ethics statement
All analyses and their reporting followed the SEER reporting guidelines. Due to the anonymously coded design of the SEER database, study‐ specific Institutional Review Board ethics approval as not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morra, S., Piccinelli, M.L., Cano Garcia, C. et al. Differences in future life expectancy of testicular germ-cell tumor patients vs. age-matched male population-based controls. Int Urol Nephrol 55, 3119–3128 (2023). https://doi.org/10.1007/s11255-023-03763-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03763-2