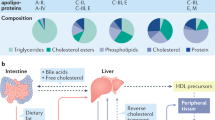
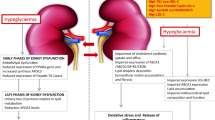
Abstract
The major cause of death among chronic kidney disease patients is cardiovascular diseases. Cardiovascular and kidney disease are interrelated and increase the severity of each other. Dyslipidemia is one the major causes of cardiovascular disease among chronic kidney disease patients along with diabetes and hypertension. The relationship between dyslipidemia and chronic kidney disease is reciprocal. Dyslipidemia is known to be a risk factor for chronic kidney disease and chronic kidney disease causes major alterations on lipoprotein profile, defined as the “dyslipidemic profile” of chronic kidney disease patients. Increased triglyceride, very low density lipoprotein and oxidized low density lipoprotein as well as decreased high density lipoprotein and changes in the composition of lipoproteins contribute to the “dyslipidemic profile.” Treatment strategies targeting the “dyslipidemic profile” of chronic kidney disease could contribute to prevent cardiovascular diseases. Current therapy is based on the patient kidney function and consist mainly of statins. This review focuses on the effects of chronic kidney disease on the lipoprotein profile and how this may impact novel therapeutic approaches to cardiovascular risk.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Thomas B et al (2017) Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28(7):2167–2179
Zubovic SV et al (2016) Chronic kidney disease and lipid disorders. Med Arch 70(3):191–192
Vanholder R et al (2016) Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diab Endocrinol 4(4):360–373
Ortiz A et al (2014) Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383(9931):1831–1843
Lamprea-Montealegre JA et al (2018) Coronary heart disease risk associated with the dyslipidaemia of chronic kidney disease. Heart
Bulbul MC et al (2018) Disorders of lipid metabolism in chronic kidney disease. Blood Purif 46(2):144–152
Huang LH, Elvington A, Randolph GJ (2015) The role of the lymphatic system in cholesterol transport. Front Pharmacol 6:182
Ossoli A, Pavanello C, Calabresi L (2016) High-density lipoprotein, lecithin: cholesterol acyltransferase, and atherosclerosis. Endocrinol Metab (Seoul) 31(2):223–229
Miida T et al (2003) LCAT-dependent conversion of prebeta1-HDL into alpha-migrating HDL is severely delayed in hemodialysis patients. J Am Soc Nephrol 14(3):732–738
Speer T, Zewinger S, Fliser D (2013) Uraemic dyslipidaemia revisited: role of high-density lipoprotein. Nephrol Dial Transplant 28(10):2456–2463
Florvall G, Basu S, Larsson A (2006) Apolipoprotein A1 is a stronger prognostic marker than are HDL and LDL cholesterol for cardiovascular disease and mortality in elderly men. J Gerontol A Biol Sci Med Sci 61(12):1262–1266
Moradi H et al (2009) Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res 153(2):77–85
Moradi H et al (2010) Increased monocyte adhesion-promoting capacity of plasma in end-stage renal disease—response to antioxidant therapy. Clin Nephrol 74(4):273–281
Goek ON et al (2012) Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol Dial Transplant 27(7):2839–2847
Zhan X et al (2018) Apolipoprotein B/apolipoprotein A1 ratio and mortality among incident peritoneal dialysis patients. Lipids Health Dis 17(1):117
Lamprea-Montealegre JA et al (2014) Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis 234(1):42–46
Wolfrum C, Poy MN, Stoffel M (2005) Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med 11(4):418–422
Sorensen IM et al (2018) Apolipoprotein M in patients with chronic kidney disease. Atherosclerosis 275:304–311
Gluba-Brzozka A et al (2017) Do HDL and LDL subfractions play a role in atherosclerosis in end-stage renal disease (ESRD) patients? Int Urol Nephrol 49(1):155–164
Rysz-Gorzynska M, Gluba-Brzozka A, Banach M (2017) High-density lipoprotein and low-density lipoprotein subfractions in patients with chronic kidney disease. Curr Vasc Pharmacol 15(2):144–151
Asztalos BF et al (2004) High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol 24(11):2181–2187
Kwiterovich PO Jr (2000) The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review. Am J Cardiol 86(12A):5L–10L
Rubinow KB et al (2017) Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int 92(6):1526–1535
Xiong X et al (2015) The association of HDL-apoCIII with coronary heart disease and the effect of statin treatment on it. Lipids Health Dis 14:127
Riwanto M et al (2013) Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 127(8):891–904
Luo M et al (2017) ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci Rep 7(1):2312
Rocha M et al (2013) Association of serum retinol binding protein 4 with atherogenic dyslipidemia in morbid obese patients. PLoS ONE 8(11):e78670
Axelsson J et al (2009) Serum retinol-binding protein concentration and its association with components of the uremic metabolic syndrome in nondiabetic patients with chronic kidney disease stage 5. Am J Nephrol 29(5):447–453
Wang K et al (2018) Alteration of HDL Protein Composition with Hemodialysis Initiation. Clin J Am Soc Nephrol 13(8):1225–1233
Sutter I et al (2015) Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis 241(2):539–546
Maeba R et al (2018) Association of cholesterol efflux capacity with plasmalogen levels of high-density lipoprotein: a cross-sectional study in chronic kidney disease patients. Atherosclerosis 270:102–109
Levkau B (2015) HDL-S1P: cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol 6:243
Nofer JR et al (2004) HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 113(4):569–581
Prufer N, Kleuser B, van der Giet M (2015) The role of serum amyloid A and sphingosine-1-phosphate on high-density lipoprotein functionality. Biol Chem 396(6–7):573–583
Kimura T et al (2006) Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem 281(49):37457–37467
Sattler K, Levkau B (2009) Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res 82(2):201–211
Brinck JW et al (2018) High-density lipoprotein from end-stage renal disease patients exhibits superior cardioprotection and increase in sphingosine-1-phosphate. Eur J Clin Invest 48(2):e12866
Vaziri ND, Liang K, Parks JS (2001) Down-regulation of hepatic lecithin: cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int 59(6):2192–2196
Calabresi L et al (2015) Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med 277(5):552–561
Miljkovic M et al (2018) Association of dyslipidemia, oxidative stress, and inflammation with redox status in VLDL, LDL, and HDL lipoproteins in patients with renal disease. Angiology 69(10):861–870
https://www.ncbi.nlm.nih.gov/gene/5446. Accessed 24 Nov 2018
Chistiakov DA et al (2017) Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 132:19–27
Umemoto T et al (2013) Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ Res 112(10):1345–1354
Vaziri ND et al (2011) Salutary effects of hemodialysis on low-density lipoprotein proinflammatory and high-density lipoprotein anti-inflammatory properties in patient with end-stage renal disease. J Natl Med Assoc 103(6):524–533
Weichhart T et al (2012) Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23(5):934–947
Tolle M et al (2012) High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res 94(1):154–162
Mao JY et al (2017) Serum amyloid A enrichment impairs the anti-inflammatory ability of HDL from diabetic nephropathy patients. J Diabetes Complications 31(10):1538–1543
Heine GH et al (2012) Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol 8(6):362–369
Ganda A et al (2013) Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 127(9):988–996
Rogacev KS et al (2014) Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14 + + CD16 + monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler Thromb Vasc Biol 34(9):2120–2127
Zhang Y et al (2016) Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med 48(5):305–312
Cetin MS et al (2016) Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ 25(11):1077–1086
Kanbay M et al (2014) Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 46(8):1619–1625
Speer T et al (2013) Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38(4):754–768
Zewinger S et al (2017) Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J 38(20):1597–1607
Shroff R et al (2014) HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol 25(11):2658–2668
Musliner TA, Michenfelder HJ, Krauss RM (1988) Interactions of high density lipoproteins with very low and low density lipoproteins during lipolysis. J Lipid Res 29(3):349–361
Cwiklinska A et al (2018) Progression of chronic kidney disease affects HDL impact on lipoprotein lipase (LPL)-mediated VLDL lipolysis efficiency. Kidney Blood Press Res 43(3):970–978
Zewinger S et al (2014) HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25(5):1073–1082
Moradi H et al (2014) Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant 29(8):1554–1562
Bowe B et al (2016) High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. veterans. Clin J Am Soc Nephrol 11(10):1784–1793
Chang TI et al. (2018) Inverse association between serum non-high-density lipoprotein cholesterol levels and mortality in patients undergoing incident hemodialysis. J Am Heart Assoc 7(12):e009096
Zewinger S et al (2015) Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J 36(43):3007–3016
Feingold KR, Grunfeld C (2000) Introduction to lipids and lipoproteins. In: De Groot LJ et al (eds) Endotext. MDText.com, Inc., South Dartmouth (MA)
Shiffman D et al (2017) LDL subfractions are associated with incident cardiovascular disease in the Malmo Prevention Project Study. Atherosclerosis 263:287–292
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18(1):29–40
Chang KC et al (2015) Increased LDL electronegativity in chronic kidney disease disrupts calcium homeostasis resulting in cardiac dysfunction. J Mol Cell Cardiol 84:36–44
Yang TC, Chang PY, Lu SC (2017) L5-LDL from ST-elevation myocardial infarction patients induces IL-1beta production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am J Physiol Heart Circ Physiol 312(2):H265–H274
Li D, Mehta JL (2005) Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res 68(3):353–354
Napoli C et al (2000) Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB J 14(13):1996–2007
Weiner DE, Sarnak MJ (2004) Managing dyslipidemia in chronic kidney disease. J Gen Intern Med 19(10):1045–1052
Tavridou A et al (2015) Association of plasma adiponectin and oxidized low-density lipoprotein with carotid intima-media thickness in diabetic nephropathy. J Diabetes Res 2015:507265
Samouilidou EC et al (2012) Lipid abnormalities and oxidized LDL in chronic kidney disease patients on hemodialysis and peritoneal dialysis. Ren Fail 34(2):160–164
Ribeiro S et al (2012) Oxidized low-density lipoprotein and lipoprotein(a) levels in chronic kidney disease patients under hemodialysis: influence of adiponectin and of a polymorphism in the apolipoprotein(a) gene. Hemodial Int 16(4):481–490
Anber V et al (1996) Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 124(2):261–271
Mohty D et al (2008) Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol 28(1):187–193
Hoogeveen RC et al (2014) Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) Study. Arterioscler Thromb Vasc Biol 34(5):1069–1077
Ivanova EA et al (2017) Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev 2017:1273042
Shen H et al (2016) Small dense low-density lipoprotein cholesterol was associated with future cardiovascular events in chronic kidney disease patients. BMC Nephrol 17(1):143
Sonmez D et al (2014) Is there a relationship between small, dense LDL and lipoprotein–associated phospholipase A2 mass in dialysis patients? Clin Lab 60(9):1431–1437
Berneis KK, Krauss RM (2002) Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 43(9):1363–1379
Sharma GS, Kumar T, Singh LR (2014) N-homocysteinylation induces different structural and functional consequences on acidic and basic proteins. PLoS ONE 9(12):e116386
Ferretti G et al (2004) Effect of homocysteinylation of low density lipoproteins on lipid peroxidation of human endothelial cells. J Cell Biochem 92(2):351–360
Ferretti G et al (2006) Homocysteinylation of low-density lipoproteins (LDL) from subjects with Type 1 diabetes: effect on oxidative damage of human endothelial cells. Diabet Med 23(7):808–813
Zinellu A et al (2010) Increased low-density lipoprotein S-homocysteinylation in chronic kidney disease. Am J Nephrol 32(3):242–248
Zinellu A et al (2012) LDL S-homocysteinylation decrease in chronic kidney disease patients undergone lipid lowering therapy. Eur J Pharm Sci 47(1):117–123
De Nicola L et al (2015) Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: multicenter prospective study in Italy. Nutr Metab Cardiovasc Dis 25(8):756–762
Visconti L et al (2016) Lipid disorders in patients with renal failure: role in cardiovascular events and progression of chronic kidney disease. J Clin Transl Endocrinol 6:8–14
Bowden RG et al (2011) Reverse epidemiology of lipid-death associations in a cohort of end-stage renal disease patients. Nephron Clin Pract 119(3):c214–9
Wanner C, Tonelli M, Improving global outcomes lipid guideline Development Work Group (2014) KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 85(6):1303–1309
Phukan RR, Goswami RK (2017) Unusual dyslipidemia in patients with chronic kidney diseases. J Clin Diagn Res 11(1):BC01–BC04
Shoji T et al (2001) Atherogenic lipoproteins in end-stage renal disease. Am J Kidney Dis 38(4 Suppl 1):S30–S33
Vaziri ND et al (2012) Lipoprotein lipase deficiency in chronic kidney disease is accompanied by down-regulation of endothelial GPIHBP1 expression. Clin Exp Nephrol 16(2):238–243
Blaton V (2009) 8. dyslipidemia at chronic renal failure. EJIFCC 20(1):59–66
Kaysen GA (2007) Hyperlipidemia in chronic kidney disease. Int J Artif Organs 30(11):987–992
Xie X et al (2017) Association of very low-density lipoprotein cholesterol with all-cause and cardiovascular mortality in peritoneal dialysis. Kidney Blood Press Res 42(1):52–61
Ooi EM et al (2011) Plasma apolipoprotein C-III metabolism in patients with chronic kidney disease. J Lipid Res 52(4):794–800
Kohan AB (2015) Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 22(2):119–125
Rocha NA et al (2017) ApoCIII as a cardiovascular risk factor and modulation by the novel lipid-lowering agent volanesorsen. Curr Atheroscler Rep 19(12):62
GA K (2006) Dyslipidemia in chronic kidney disease: causes and consequences. Kidney Int 70:S55–S58
Selmeci L et al (2005) Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: a simple, fast and inexpensive automated technique. Clin Chem Lab Med 43(3):294–297
Skvarilova M et al (2005) Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149(1):83–87
Vaziri ND (2006) Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290(2):F262–F272
Mikolasevic I et al (2017) Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis 10:35–45
Hirano T et al (2003) Very low-density lipoprotein-apoprotein CI is increased in diabetic nephropathy: comparison with apoprotein CIII. Kidney Int 63(6):2171–2177
Turak O et al (2016) The Role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict new cardiovascular events in essential hypertensive patients. J Clin Hypertens (Greenwich) 18(8):772–777
Benjannet S et al (2004) NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 279(47):48865–48875
Abifadel M et al (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34(2):154–156
Morena M et al (2017) Plasma PCSK9 concentrations during the course of nondiabetic chronic kidney disease: relationship with glomerular filtration rate and lipid metabolism. J Clin Lipidol 11(1):87–93
McCormick SP (2004) Lipoprotein(a): biology and clinical importance. Clin Biochem Rev 25(1):69–80
Hopewell JC, Haynes R, Baigent C (2018) The role of lipoprotein (a) in chronic kidney disease. J Lipid Res 59(4):577–585
Lin J et al (2015) Relation of atherogenic lipoproteins with estimated glomerular filtration rate decline: a longitudinal study. BMC Nephrol 16:130
Lin J et al (2014) Plasma lipoprotein(a) levels are associated with mild renal impairment in type 2 diabetics independent of albuminuria. PLoS ONE 9(12):e114397
Konishi H et al (2016) Plasma lipoprotein(a) predicts major cardiovascular events in patients with chronic kidney disease who undergo percutaneous coronary intervention. Int J Cardiol 205:50–53
Guerraty MA et al (2015) Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol 115(9):1281–1286
Toth PP et al (2018) Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int 93(6):1397–1408
Kanbay M et al (2009) Statin treatment for dyslipidemia in chronic kidney disease and renal transplantation: a review of the evidence. J Nephrol 22(5):598–609
Trialists CT (2016) C., et al., Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 4(10):829–839
Ferro CJ et al. (2018) Lipid management in patients with chronic kidney disease. Nat Rev Nephrol
Baigent C et al (2011) The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377(9784):2181–2192
Fukumoto Y (2018) Impact of statin-ezetimibe combination in chronic kidney disease. Int J Cardiol 268:36–37
Yan YL et al (2015) High-intensity statin therapy in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ Open 5(5):e006886
Chung CM et al (2017) Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J Clin Lipidol 11(2):422–431 e2
Walther CP et al (2018) Association between intensity of statin therapy and mortality in persons with chronic kidney disease. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy237
Wanner C et al (2005) Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353(3):238–248
Chan KE et al (2010) Modeling the 4D Study: statins and cardiovascular outcomes in long-term hemodialysis patients with diabetes. Clin J Am Soc Nephrol 5(5):856–866
Fellstrom B et al (2005) Effect of rosuvastatin on outcomes in chronic haemodialysis patients—design and rationale of the AURORA study. Curr Control Trials Cardiovasc Med 6(1):9
Athyros VG et al (2009) Statins and cardiovascular events in patients with end-stage renal disease on hemodialysis. The AURORA results suggest the need for earlier intervention. Curr Vasc Pharmacol 7(3):264–266
Cho EY et al (2017) Efficacy of statin treatment in early-stage chronic kidney disease. PLoS ONE 12(1):e0170017
Scarpioni R et al (2012) Treatment of dyslipidemia in chronic kidney disease: effectiveness and safety of statins. World J Nephrol 1(6):184–194
Balk EM et al (2003) Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 139(8):670–682
Hottelart C et al (2002) Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron 92(3):536–541
McPherson R et al (2006) Canadian Cardiovascular Society position statement–recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol 22(11):913–927
Jun M et al (2012) Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 60(20):2061–2071
Weinstein DL et al (2013) A randomized, double-blind study of fenofibric acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease. Clin Ther 35(8):1186–1198
Kasiske B et al (2004) Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant 4(Suppl 7):13–53
Vanholder R et al. (2018) Deleting death and dialysis: conservative care of cardio-vascular risk and kidney function loss in chronic kidney disease (CKD). Toxins (Basel). 10(6):237
Hassan KS et al (2010) Effects of omega-3 on lipid profile and inflammation markers in peritoneal dialysis patients. Ren Fail 32(9):1031–1035
Tannock L (2000) Dyslipidemia in chronic kidney disease. In: De Groot LJ et al (eds) Endotext. MDText.com, Inc., South Dartmouth (MA)
Jin Kang H et al (2013) Effects of low-dose niacin on dyslipidemia and serum phosphorus in patients with chronic kidney disease. Kidney Res Clin Pract 32(1):21–26
Ix JH et al (2011) Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am J Kidney Dis 57(6):963–965
Malhotra R et al (2018) The effect of extended release niacin on markers of mineral metabolism in CKD. Clin J Am Soc Nephrol 13(1):36–44
Harper CR, Jacobson TA (2008) Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol 51(25):2375–2384
Kalil RS et al (2015) Effect of extended-release niacin on cardiovascular events and kidney function in chronic kidney disease: a post hoc analysis of the AIM-HIGH trial. Kidney Int 87(6):1250–1257
Stoekenbroek RM, Kastelein JJ, Huijgen R (2015) PCSK9 inhibition: the way forward in the treatment of dyslipidemia. BMC Med 13:258
Zheng-Lin B, Ortiz A (2018) Lipid management in Chronic Kidney Disease: systematic review of PCSK9 targeting. Drugs 78(2):215–229
Toth PP et al (2016) Effect of alirocumab on specific lipoprotein non-high-density lipoprotein cholesterol and subfractions as measured by the vertical auto profile method: analysis of 3 randomized trials versus placebo. Lipids Health Dis 15:28
Mafham M, Haynes R (2018) PCSK9 inhibition: ready for prime time in CKD? Kidney Int 93(6):1267–1269
Elewa U et al (2016) PCSK9 in diabetic kidney disease. Eur J Clin Invest 46(9):779–786
Barcellos FC et al (2015) Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 8(6):753–765
Miele EM et al (2017) High-density lipoprotein particle pattern and overall lipid responses to a short-term moderate-intensity aerobic exercise training intervention in patients with chronic kidney disease. Clin Kidney J 10(4):524–531
Afsar B et al (2014) An update on coronary artery disease and chronic kidney disease. Int J Nephrol 2014:767424
Shen H et al (2018) Effects of statin therapy on chronic kidney disease patients with coronary artery disease. Lipids Health Dis 17(1):84
Acknowledgements
MK gratefully acknowledges use of the services and facilities of the KoC University Research Center for Translational Medicine (KUTTAM), funded by the Republic of Turkey Ministry of Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Ministry of Development. AO was supported by FIS PI16/02057, ISCIII-RETIC REDinREN RD016/0009 FEDER funds, Sociedad Española de Nefrología, Fundacion Renal Iñigo Álvarez de Toledo (FRIAT), Comunidad de Madrid Biomedicina B2017/BMD-3686 CIFRA2-CM.
Funding
This study was not funded by any Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Dincer, N., Dagel, T., Afsar, B. et al. The effect of chronic kidney disease on lipid metabolism. Int Urol Nephrol 51, 265–277 (2019). https://doi.org/10.1007/s11255-018-2047-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-2047-y
Keywords
Profiles
- Mehmet Kanbay View author profile