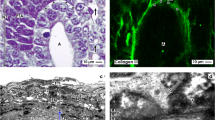
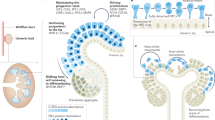
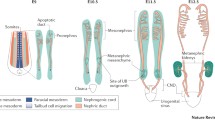
Abstract
The development of the mammalian kidney is a complex and in part unknown process which requires interactions between pluripotential/stem cells, undifferentiated mesenchymal cells, epithelial and mesenchymal components, eventually leading to the coordinate development of multiple different specialized epithelial, endothelial and stromal cell types within the kidney architectural complexity. We will describe the embryology and molecular nephrogenetic mechanisms, a fascinating traffic of cells and tissues which takes place in five stages: (1) ureteric bud (UB) development; (2) cap mesenchyme formation; (3) mesenchymal–epithelial transition (MET); (4) glomerulogenesis and tubulogenesis; (5) interstitial cell development. In particular, we will analyze the multiple cell types involved in these dramatic events as characters moving between different worlds, from the mesenchymal to the epithelial world and back, and will start to define the multiple factors that propel these cells during their travels throughout the developing kidney. Moreover, according with the hypothesis of renal perinatal programing, we will present the results reached in the fields of immunohistochemistry and molecular biology, by means of which we can explain how a loss or excess of molecular factors governing nephrogenesis may cause the onset of pathologies of different gravity, in some cases leading to a chronic kidney disease at different times from birth.


Similar content being viewed by others
References
Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P et al (2011) Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol 227:1257–1268
Fanni D, Gerosa C, Nemolato S, Mocci C, Pichiri G, Coni P et al (2012) “Physiological” renal regenerating medicine in VLBW preterm infants: could a dream come true? J Matern Fetal Neonatal Med 25(3):41–48
Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH (2008) Larsen’s human embryology. Churchill Livingstone/Elsevier, Philadelphia
Watanabe T, Costantini F (2004) Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol 271:98–108
Yosypiv IV (2008) A new role for the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Keio J Med 57:184–189
Reidy K, Rosenblum N (2009) Cell and molecular biology of kidney development. Semin Nephrol 29:321–337
Michos O, Cebrian C, Hyink D, Grieshamer U, Williams L, D’Agati V et al (2010) Kidney development in the absence of Gdnf and Spry I requires Fgf10. PLoS Genet 6:e1000809
Moritz K, Wintour E, Black M, Bertram J, Caruana G (2008) Factors influencing mammalian kidney development: implications for health in adult life. Adv Anat Embryol Cell Biol 196:1–78
Jain S (2009) The many faces of RET dysfunction in kidney. Organogenesis 5:177–190
Zhang M, Wang J, Cheng H, Harris R (1997) McKanna. cyclooxygenase-2 in rat nephron development. Am J Physiol 273:F994–F1002
Schedl A, Reginensi A, Clarkson M, Lu B.(2010) Role of SOX genes during kidney development. In: 11th International Workshop on Developmental Nephrology Proceedings, New York, Abstract O-30
Basson M, Akbulut S, Watson-Johnson J, Simon R, Carroll T, Shakya R et al (2005) Sprouty 1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8:229–239
Miyazaki Y, Oshima K, Fogo A, Hogan B, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105:863–873
Reidy K, Villegas G, Teichman J, Veron D, Shen W, Jimenez JT et al (2009) Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development 136:3979–3989
Marose T, Merkel C, McMahon A, Carroll T (2008) Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol 314:112–126
Grote D, Souabni A, Busslinger M, Bouchard M (2006) Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133:53–61
Hopkins C, Ineson J, Little M.(2010) Functional conversion of the human kidney epithelial cells to renal progenitors requires more than generalized epigenetic destabilization. In: 11th International Workshop on Developmental Nephrology Proceedings, New York, Abstract P-6
Welham S, Riley P, Wade A, Hubank M, Woolf A (2005) Maternal diet programs embryonic kidney gene expression. Physiol Genomics 22:48–56
Singh R, Moritz K, Bertram J, Cullen-McEwen L (2007) Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol 293:F548–F554
Simeoni U, Ligi I, Buffat C, Boubred F (2010) Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatr Nephrol. doi:10.1007/s00467-010-1648-1
Cohen T, Loutochin O, Amin M, Capolicchio J, Goodyer P, Jednak R (2007) PAX2 is reactivated in urinary tract obstruction and partially protects collecting duct cells from programmed cell death. Am J Physiol Renal Physiol 292:F1267–F1273
Benetti E, Artifoni L, Salviati L, Pinello L, Perrotta S, Zuffardi O, Zacchello G, Murer L (2007) Renal hypoplasia without optic coloboma associated with PAX2 gene detection. Nephrol Dial Transplant 22(7):2076–2078
Salomon R, Tellier AL, Attie-Bitach T et al (2001) PAX2 mutations in oligomeganephronia. Kidney Int 59:457–462
Fletcher J, Hu M, Berman Y et al (2005) Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J Am Soc Nephrol 16:2754–2761
Weber S, Moriniere V, Knuppel T et al (2006) Prevalence of mutations in renal developmental genes in children with renal hypoplasia: results of the ESCAPE study. J Am Soc Nephrol 17:2864–2870
Schimmenti LA, Cunliffe HE, McNoe LA et al (1997) Further delineation of renal-coloboma syndrome in patients with remarkable variability of phenotype and identical PAX2 mutations. Am J Hum Genet 60:869–878
Scott JE, Renwick M (1988) Antenatal diagnosis of congenital abnormalities in the urinary tract. Results from the Northern Region Fetal Abnormality Survey. Br J Urol 62:295–300
Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA 3rd, Hogan BM, Fogo A, Brock JW 3rd, Inagami T, Ichikawa I (1999) Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 3:1–10
Niimura F, Kon V, Ichikawa I (2006) The renin-angiotensin system in the development of the congenital anomalies of the kidney and urinary tract. Curr Opin Pediatr 18:161–166
Perantoni A, Dove L, Karavanova I (1995) Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92:4696–4700
Poladia D, Kish K, Kutay B, Hains D, Kegg H, Zhao H et al (2006) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291:325–339
Lelongt B, Trugnan G, Murphy G, Ronco P (1997) Matrix metalloproteinases MMP2 and MMP9are produced in early stages of kidney morphogenesis but onlyMMP9is required for renal organogenesis in vitro. J Cell Biol 136:1363–1373
Arnould C, Lelièvre-Pégorier M, Ronco P, Lelongt B (2009) MMP9 limits apoptosis and stimulates branching morphogenesis during kidney development. J Am Soc Nephrol 20(10):2171–2180
Kuure S, Cebrian C, Machingo Q, Lu B, Chi X, Hyink D et al (2010) Actin depolymerizing factors Cofilin1 and Destrin are required for ureteric bud branching morphogenesis. PLoS Genet 6:e1001176
Rosenblum N (2008) Developmental biology of the human kidney. Semin Nephrol 13:125–132
Boyle S, Misfeldt A, Chandler K, Deal K, Southard-Smith E, Mortlock D et al (2008) Fate mapping using Cited I-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313:234–245
Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R (2006) Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 133:151–161
Kobayashi A, Valerius M, Mugford J, Carroll T, Self M, Oliver G et al (2008) Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3:169–181
Nishinakamura R, Takasato M (2005) Essential roles of Sall1 in kidney development. Kidney Int 68:1948–1950
Weber S, Moriniere V, Knuppel T et al (2006) Prevalence of mutations in renal developmental genes in children with renal hypoplasia: results of the ESCAPE study. J Am Soc Nephrol 17:2864–2870
Townes PL, Brocks ER (1972) Hereditary syndrome of imperforate anus with hand, foot and ear anomalies. J Pediatr 8:321–326
Serville F, Lacombe D, Saura R, Billeaud C, Sergent MP (1993) Townes–Brocks syndrome in an infant with translocation t(5;16). Genet Couns 4:109–112
O’Callaghan M, Young ID (1995) Townes–Brocks syndrome. In: Donnai D, Winter R (eds) Congenital malformation syndromes. London, Chapman and Hall, pp 326–332
Wischermann A, Holschneider AM (1997) Townes–Brocks-syndrome. Monatsschr Kinderheilkd. 145:382–386
Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C, Breuning MH, ten Kate LP, Maaswinkel-Mooy P, Mitulla B et al (1999) Molecular analysis of SALL1 mutations in Townes–Brocks syndrome. Am J Hum Genet 64:435–445
Mugford J, Yu J, Kobayashi A, McMahon A (2009) High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol 333:312–323
Mudumana S, Hentschel D, Liu Y, Vasilyev A, Drummond I (2008) Odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development 135(20):3355–3367
Self M, Lagutin O, Bowling B, Hendrix J, Cai Y, Dressler G et al (2006) Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25:5214–5228
Kreidberg J (2010) WT1 and kidney progenitor cells. Organogenesis 6:61–70
Call KM et al (1990) Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 60:509–520
Gessler M et al (1990) Homozygous deletion in Wilms’ tumor of a zinc-finger gene identified by chromosome jumping. Nature 343:774–778
Pritchard-Jones K et al (1990) The candidate Wilms’ tumour gene is involved in genitourinary development. Nature 346:194–197
Fanni D, Fanos V, Monga G, Gerosa C, Locci A, Nemolato S et al (2011) Expression of WT1 during normal human kidney development. J Matern Fetal Neonatal Med 24(2):45–48
Luo G, Hofmann C, Bronckers A, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820
Palmer R, Kotsianti A, Cadman B, Boyd T, Gerald W, Haber D (2001) WT1 regulates the expression of the major glomerular podocyte membrane protein Podocalyxin. Curr Biol 11(22):1805–1809
Wagner N, Wagner K, Xing Y, Scholz H, Schedl A (2004) The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol 15(12):3044–3051
Ryan G, Steele-Perkins V, Morris J, Rauscher F 3rd, Dressler GR (1995) Repression of PAX-2 by WT1 during normal kidney development. Development 121(3):867–875
Chugh SS (2007) Transcriptional regulation of podocyte disease. Transl Res 149:237–242
Pelletier J, Bruening W, Kashtan C, Mauer S, Manivel J, Striegel J et al (1991) Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys–Drash syndrome. Cell 67:437–447
Barbaux S, Niaudet P, Gubler M, Grunfeld J, Jaubert F, Kuttenn F et al (1997) Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17:467–470
Barisoni L, Kriz W, Mundel P, D’Agati V (1999) The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10:51–61
Orloff MS, Iyengar SK, Winkler CA, Goddard KA, Dart RA, Ahuja TS et al (2005) Variants in the Wilms’ tumor gene are associated with focal segmental glomerulosclerosis in the African American population. Physiol Genomics 21:212–221
Frasier S, Bashore RA, Mosier HD (1964) Gonadoblastoma associated with pure gonadal dysgenesis in monozygotic twins. J Pediatr 64:740–745
Haning RV, Chesney RW, Moorthy AV, Gilbert EF (1985) A syndrome of chronic renal failure and XY gonadal dysgenesis in young phenotypic females without genital ambiguity. Am J Kidney Dis 6:40–48
Kinberg JA, Angle CR, Wilson RB (1987) Nephropathy-gonadal dysgenesis, type 2: renal failure in three siblings with dysgenesis in one. Am J Kidney Dis 9:507–510
Moorthy AV, Chesney RW, Lubinsky M (1987) Chronic renal failure and XY gonadal dysgenesis: “Frasier” syndrome— commentary on reported cases. Am J Med Genet 3:297–302
Perantoni A, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C et al (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132:3859–3871
Kispert A, Vainio S, McMahon A (1998) Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125:4225–4234
Karner C, Chirumamilla R, Aoki S, Igarashi P, Wallingford J, Carroll T (2009) Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41(7):793–799
Fanni D, Fanos V, Monga G, Gerosa C, Nemolato L, Van Eyken P et al (2011) MUC1 in mesenchymal-to-epithelial transition during human nephrogenesis: changing the fate of renal progenitor/stem cells? J Matern Fetal Neonatal Med 24(2):63–66
Little M, Brennan J, Georgas K, Davies J, Davidson D, Baldock R et al (2007) A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns 7:680–699
Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T et al (2000) An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57:1836–1846
Thony H, Luethy C, Zimmermann A, Laux-End R, Oetliker O, Bianchetti M (1995) Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur J Pediatr 154:S65–S68
Bernstein J, Risdon RA (1997) Renal system. In: Gilbert-Barness E (ed) Potter’s Pathology of the Fetus and Infant. Mosby, St Louis, pp 863–866
Rodriguez M, Gomez A, Abitbol C, Chandar J, Duara S, Zilleruelo G (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25
Surendran K, Boyle S, Barak H, Kim M, Stromberski C, McCright B et al (2010) The contribution of NOTCH1to nephron segmentation in the developing kidney is revealed in a sensitized NOTCH2 background and can be augmented by reducing mint dosage. Dev Biol 337:386–390
Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont MEM, Rand EB, Piccoli DA, Hood L, Spinner NB (1997) Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16:243–251
Oda T, Elkahlous AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PM, Spinner NB, Collins FS, Chandrasekharappa SC (1997) Mutations in the human Jagged1gene are responsible for Alagille syndrome. Nat Genet 16:235–242
McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB (2006) NOTCH2 mutations cause Alagille Syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet 79(1):169–173
Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, Dommergues JP (1987) Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr 110:195–200
Harendza S, Hubner CA, Glaser C, Burdelski M, Thaiss F, Hansmann I, Gal A, Stahl RAK (2005) Renal failure and hypertension in Alagille syndrome with a novel JAG1 mutation. J Nephrol 18:312–317
Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA (1999) Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 29:822–829
Fonseca Ferraz M, Dos Santos AC, Rossi R, Correa R, Dos Reis M, de Paula Antunes Teixeira V (2008) Histochemical and immunohistochemical study of the glomerular development in human fetuses. Pediatr Nephrol 23:257–262
Reidy K, Tufro A (2011) Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol 26:1407–1412
Faa G, Gerosa C, Fanni D, Nemolato S, Marinelli V, Locci A et al (2011) CD10 in the developing human kidney: immunoreactivity and possible role in renal embryogenesis. J Matern Fetal Neonatal Med 25(7):904–911
Papandreu CN, Nanus DM (2010) Is methylation the key to CD10 loss? J Pediatr Hematol Oncol 32:2–3
Avery AK, Beckstead J, Renshaw AA, Corless CL (2000) Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol 24:203–210
Faa G, Gerosa C, Fanni D, Nemolato S, Monga G, Fanos V. Kidney embryogenesis: how to look at old things with new eyes. In: Fanos V, Chevalier RL, Faa G, Cataldi L (2011) Developmental Nephrology: From Embryology to Metabolomics, Chapter 1. Hygeia Press, Quartu S. Elena (Ca), Italy, pp 21–45
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vassilios Fanos and Cristina Loddo have contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Fanos, V., Loddo, C., Puddu, M. et al. From ureteric bud to the first glomeruli: genes, mediators, kidney alterations. Int Urol Nephrol 47, 109–116 (2015). https://doi.org/10.1007/s11255-014-0784-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0784-0