Abstract
Urban environments present wildlife with major challenges and yet surprising numbers of species have colonised towns and cities globally. Despite the growing realisation that urban centres can be important habitats for wildlife, why some species do better than others in urban environments remains poorly understood. Here, we compare the breeding performance of an apex predator, the peregrine falcon (Falco peregrinus), in urban and rural environments, and test whether variation in reproductive success between and within environments is driven by prey. Historical breeding data were collected from raptor study groups across Great Britain between 2006 and 2016, from 22 urban and 58 rural nest sites, involving 101 and 326 nesting attempts, respectively. Prey density, biomass and diversity around the individual nests was estimated using modelled estimates from a national bird census. Urban peregrines produced more fledglings and had a higher overall nesting success (i.e. whether a nesting attempt was successful or unsuccessful) than rural peregrines. Prey density and biomass were significantly higher, and diversity significantly lower, in the urban sites, and explained the variation in reproductive success within both the urban and rural environments. Therefore, urban environments in Great Britain appear to provide peregrine falcons with superior habitats in terms of prey availability compared to rural habitats. We conclude that some apex predators can benefit from urban environments and that urban planning has the potential to benefit biodiversity across many trophic levels.
Similar content being viewed by others
Introduction
Urbanisation is increasing rapidly, causing profound and irreversible changes to natural landscapes (Gaston 2010). These changes represent major challenges for some wildlife populations through associated habitat loss and fragmentation (McKinney 2006), barriers to dispersal (Erritzoe et al. 2003; Bishop and Brogan 2013), increased disease (Dhondt et al. 2007), noise and light pollution (Fuller et al. 2007; Kempenaers et al. 2010), human disturbance (Schlesinger et al. 2008), increased mortality due to road traffic accidents and collisions (Erritzoe et al. 2003), and high densities of domesticated predators such as cats (Felis catus) (Schlesinger et al. 2008). Although many species are unable to persist in urban environments, others are able to colonise and reproduce successfully in even the most extreme urban environments (Blair 1996; Marzluff 2001). Why this variation between species occurs has become a major question in current ecology (e.g. Brand and Snodgrass 2010; Bonnington et al. 2015; Orros et al. 2015; Russo and Ancillotto 2015; Demeyrier et al. 2016; Rautio et al. 2016).
Key factors that are likely to explain how well species can persist in urban environments include whether the species is a specialist or a generalist, how well they tolerate human disturbance, the availability of suitable habitat, their exposure to predation, and food availability (Shultz et al. 2005; Fuller et al. 2008; Sims et al. 2008; Evans et al. 2009, 2010, 2011; Pettett et al. 2017). Even when species persist and manage to reproduce in urban environments, their breeding performance relative to traditional habitats can be highly variable across and within species (e.g. Chamberlain et al. 2009; Kettel et al. 2018). In some cases, urban populations do worse than those in traditional environments (e.g. Mennechez and Clergeau 2006; Peach et al. 2008; Pollock et al. 2017). In others they are just as successful (e.g. Conway et al. 2006; Suri et al. 2017) and in others, still, urban do better than traditional populations (e.g. Solonen 2008; Rebolo-Ifrán et al. 2017). It is suggested that higher temperatures and food availability, and a reduced level of predation from natural predators, are likely to be the key reasons why populations sometimes do better in urban environments (e.g. Newhouse et al. 2008). The generality of this conclusion remains unclear, however, not least because most research in this area comes from low trophic-level species. Higher trophic-level (apex) predatory species are likely to show very different patterns because they often have large home ranges and require an abundance of suitable prey, factors not often associated with urban environments (Fischer et al. 2012). However, some mammalian, avian and reptile (notably snake) predators have exploited urban environments very effectively (Fischer et al. 2012), where their densities can be relatively high when food availability is high (Ordeñana et al. 2010; Šálek et al. 2014). Urban centres are often associated with an abundant and stable food supply in the form of discarded human food (Maciusik et al. 2010), or food that is deliberately provided in gardens and greenspaces (Jones and Reynolds 2008; Rautio et al. 2016). Nevertheless, the predictability of food may be harder in urban environments, and the quality of food may not necessarily be optimal (e.g. Heiss et al. 2012; Meillère et al. 2015). Consequently, clear evidence that differences in prey availability may be driving the high reproductive success sometimes reported among urban predators is lacking (Bateman and Fleming 2012).
Avian predators provide a good model for understanding the effects of urbanisation because they are readily detectable and their ecology is generally well known. Kettel et al. (2018) highlight that while the breeding performance of small mammal-eating raptors tends to be reduced in urban environments (e.g. Tella et al. 1996; Liven-Schulman et al. 2004; Charter et al. 2007), the opposite is true for bird-eating raptors (e.g. Boal and Mannan 1999; Solonen 2008; Lin et al. 2015). Again, it has been suggested that prey availability plays a key role because native small mammal densities are known to decline with urbanisation (Baker et al. 2003), whilst bird densities increase (Blair 1996; Tratalos et al. 2007). Indeed, low small-mammal prey availability has been linked to reduced breeding performance in urban predators (Sumasgutner et al. 2014), and high avian prey availability is thought to have a positive effect on the health of urban raptor nestlings (Suri et al. 2017). To date, however, no studies have directly linked high avian prey density to an improved breeding performance of an urban predator.
Our aim was to examine the breeding performance of an apex predator, the peregrine falcon (Falco peregrinus; hereafter ‘peregrine’), in urban and rural environments in relation to prey availability. Peregrines are specialist bird-eating raptors found in cities globally (Altwegg et al. 2014; Wilson et al. 2018), an environment in which some potential prey species of peregrines are especially abundant. Despite being one of the best-known urban predators, it is unclear whether the peregrine is benefitting from nesting in urban environments. We predicted that breeding success would be relatively high in urban environments and that this would be explained by higher prey availability in urban centres.
Methods
Breeding data
Reproductive success data were collected from 2006 – 2016 by amateur raptor and bird study groups, conservation organisations, peregrine researchers, and organisations involved in the maintenance of peregrine web-cameras (in urban locations). Data comprised 101 nesting attempts over 22 nest sites in urban environments, and 326 nesting attempts over 58 sites in rural environments. Data for urban nests were less extensive due to the relatively recent colonisation of urban areas in the UK by peregrines (Wilson et al. 2018). ‘Urban’ sites were defined as nests located in towns or cities, and contained at least 50% urban or suburban land-cover (using the Land Cover Map 2007) within a 2-km radius of the nest. ‘Rural’ sites included inland, natural nest-sites or quarries (either used or disused), outside of towns or cityscapes, and containing no more than 10% urban or suburban land-cover within a 2-km radius of the nest. Sites located on grouse moorland, where persecution leads to reduced breeding performance (Amar et al. 2012), were excluded. Data obtained included nest location and number of young fledged (to reach fledging age or actually leave the nest), for each nesting attempt.
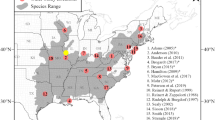
Urban nests (n = 22) were located across England (n = 21), with one site in Wales, whereas rural nests (n = 58) were only located in Derbyshire (n = 21), Gloucestershire (n = 13), Leicestershire (n = 1), Shropshire (n = 22) and Staffordshire (n = 1), in England (Fig. 1). The disparity in nest locations across regions is explained in part by the natural distribution of peregrines. For example, in low-lying eastern England natural nest sites are relatively rare (Ratcliffe 2010; Balmer et al. 2013).
The counties in England and Wales where peregrine breeding data were obtained. Light grey shading shows where data for rural peregrines were obtained, dark grey shading shows where data for urban peregrines were obtained, and black shading shows where data for both urban and rural peregrines were obtained. Sample sizes for urban (u) and rural (r) are shown for each county.
Prey data
Here we use the term ‘prey data’ to refer generally to prey density, prey biomass and prey diversity, collectively. Prey data were gathered for each peregrine nest using data from the Breeding Bird Survey (BBS) (Harris et al. 2016) because it provides a smaller-scale (1-km) resolution than other national surveys (e.g. Bird Atlas). The BBS is a volunteer-based annual survey that is organised by the British Trust for Ornithology (BTO) and aims to monitor population trends of the UK's breeding birds. Volunteers visit randomly-allocated 1-km squares during the breeding season, recording all birds heard or seen along two parallel transects within the square. Few of the squares surveyed overlapped with the peregrine sites; therefore, modelled densities provided by the BTO (following Massimino et al. 2015), based on BBS data from 2007 and 2009, were used as our prey densities, and we assumed that modelled densities reflected the raw data accurately. We also assumed this partial 3-year temporal overlap with the breeding data led to no bias in the patterns reported below. The predicted density (hereafter ‘density’) of each prey species per 1-km grid was estimated using Generalised Additive Models with land-use, elevation, and eastings and northings (to account for spatial variation across different regions) as explanatory factors (full methodology described in Massimino et al. 2015). Though the peregrine’s diet can be dominated by medium-sized prey, such as feral pigeons (Columba livia) and wader species, they are generalist predators of birds and often take prey from the smallest species up to 500g (Drewitt and Dixon 2008; Ratcliffe 2010). We therefore chose to include the density of 49 individual bird species known to be depredated by peregrines (Ratcliffe 2010; Table 1). Data were only available for these 49 species as they were the most common birds observed during the BBS, providing enough data to model their density (Massimino, pers. comms.). Densities were calculated for each 1-km grid square contained within a 2-km radius of a known peregrine nest. All urban nests were included in analyses but volunteers provided the exact locations of only 27 rural nests (because of the sensitivity of the data), reducing the sample. A 2-km radius was chosen as this is considered to be the approximate core range of a breeding female peregrine in GB (Mearns 1985; Ratcliffe 2010). The 2-km radius included all 1-km squares within, and touching, the boundary around the peregrine’s nest. Thus, the number of 1-km squares varied slightly among nest sites.
The total biomass of each prey species per 1-km grid square was calculated by multiplying the expected density estimates by average species biomass (from Snow and Perrins 1997) for each of the 49 species. The low sampling resolution of the species data did not allow analyses for individual species or groups of species to be conducted; thus, data were summed to produce a total prey biomass estimate for the nest area. Prey diversity in each 1-km grid square within the 2-km radius was also calculated using Simpson’s diversity index, which takes into account both species richness and abundance (Simpson 1949). Overall density (i.e. the number of all birds of all 49 species per 1-km grid square), biomass and diversity were calculated by taking the average of the values obtained for each of the 1-km grid squares within the 2-km radius of each site, giving an average value per 1-km for each site.
Statistical analyses
Breeding performance across urban and rural landscapes
Generalised linear mixed models (GLMMs) were used to test the effect of landscape type (urban or rural) and geographical location (county), which we took to represent a range of measures including regional climate, on three breeding parameter responses. Number of fledglings from all nesting attempts (i.e. including sites that attempted to fledge young but may or may not have produced young to fledge in a given year), number of fledglings from successful nesting attempts only (i.e. sites that only produced at least one young to fledge), and whether the nesting attempt was successful (binary response: successful or unsuccessful), were fitted as response variables in separate models. Year and site were fitted as random terms in each model, and landscape type and county were fitted as fixed effects. Interactive terms between landscape type and county could not be fitted as only three out of the fifteen counties had data for both urban and rural sites. Binomial error structures with a probit link function were used to test the probability of nest success. Quasi-Poisson error structures with a log link function were used to test for effects on number to fledge from all nesting attempts and number to fledge from successful nesting attempts. Quasi-Poisson structures were chosen to control for overdispersion.
Variation in prey across urban and rural landscapes
A principal component analysis was carried out to reduce the dimensionality of the interrelated variables related to prey (prey density, prey biomass and prey diversity), creating a single new prey component that captured 77.5% of the variance amongst the variables. This component correlated positively with prey density and prey biomass, and negatively with prey diversity. Hence, it can be interpreted as a measure of ‘prey availability’.
A general linear model (GLM) with a log link function was fitted to test the effect of landscape type (urban or rural) on the prey component, where landscape type was fitted as a fixed effect.
Effects of prey on peregrine breeding performance
GLMMs were fitted to test the effects of the prey component on breeding performance. Both the number of fledglings from successful nests (Poisson error structure with sqrt link function) and nest success (binomial error structure with probit link function), were fitted as response variables in two different models. The prey component and landscape type were fitted as fixed effects in each model, with year and site fitted as random terms. Interactive terms between landscape type (urban or rural) and the prey component were also included in the models.
To obtain the best fitting models, the error distributions and link functions of all models were changed and compared using an information theoretic approach via AIC comparison. The model with the lowest AIC value was chosen as the best fitting model. All statistical analyses were performed using R (version 3.2.2).
Results
Breeding performance in urban and rural landscapes
Peregrines nesting in urban sites produced approximately one more chick to fledge from all nesting attempts, and from successful nesting attempts only, than those nesting in rural sites (Table 2; Fig. 2a). Furthermore, the probability that a nesting attempt would be successful was higher at urban sites (94 % successful) compared to rural sites (78 % successful; Table 2; Fig. 2b). There was no effect of county on any of the breeding parameters, suggesting climate or other local factors was unlikely to cause the difference between breeding performance in urban and rural sites (Table 2).
Mean (±1 SE) number of chicks to fledge from (a) successful nests only, number of chicks to fledge from all nesting attempts and (b) percentage of successful nesting attempts at urban and rural sites between 2006 and 2016. Sample sizes are shown in Table 2
Variation in prey availability across urban and rural landscapes
There was a significant effect of landscape type on the prey component (X2(304) = 12128, p < 0.001), where urban sites had a higher prey component (i.e. high prey density and biomass, but low prey diversity per 1-km2) compared to rural sites (Fig. 3).
Breeding performance in relation to prey
The prey component had a significant positive effect on the number of young to fledge (X2(304) = 320.63, p < 0.001; Fig. 4a); i.e. the higher the prey density and biomass, and lower the prey diversity per 1-km2, the greater the number of young to fledge. There was no significant interaction between landscape type and the prey component (X2(304) = 99.61, p = 0.66) on the number of young to fledge.
Mean (±1 SE) prey component per 1-km2 within a 2-km radius of peregrine nests in relation to (a) number of fledglings per successful nesting attempt and (b) nest success at urban and rural nests combined. The higher the number for prey availability, the higher the prey density and biomass, and the lower the prey diversity
The prey component also had a significant positive effect on the probability of nest success (X2(304) = 253.83, p < 0.001; Fig. 4b). Again, there was no significant interaction between landscape type and the prey component (X2(304) = 250.47, p = 0.30) on nest success.
Discussion
Here, we provide evidence that the breeding performance of an apex predator is positively influenced by urbanisation on a national scale. Peregrines in urban environments across Great Britain were more successful than those in rural environments in terms of number of fledglings and nesting success. Indeed, the success of urban peregrine nests in this study (94 %) is the highest reported for any urban raptor species (Kettel et al. 2018), or for rural peregrines in GB and elsewhere (e.g. Crick and Ratcliffe 1995; Amar et al. 2012; Burke et al. 2015). The prey component, including predicted prey density and biomass, was higher in urban sites, and was positively correlated with breeding performance. Thus our predictions were met because prey were more abundant in urban habitats as expected (Blair 1996; Tratalos et al. 2007), and it is well known that food abundance is typically the major driver of breeding performance among birds in particular (e.g. Martin 1987; Uttley et al. 1994; Perrig et al. 2014; Pollock et al. 2017) and animals in general (e.g. Korpimäki et al. 1991; Wauter and Lens 1995; Heesen et al. 2013)
Our results agree with another more detailed study based on a single urban raptor population in South Africa, which concluded that the stability of prey in urban environments is sufficient to maintain healthy offspring of another top avian predator (Suri et al. 2017). Furthermore, a study in Italy suggested higher densities of avian predators occurred in urban centres with higher densities of prey (Sorace 2002). In contrast, however, Gahbauer et al. (2015) found no difference in the number of young to fledge from urban and rural peregrines in northeastern North America, and suggested that habitat quality may have been similar across the two environments. The difference in findings between Gahbauer et al.’s (2015) and our study could arise for many reasons. First, for example, in Gahbauer et al.’s study, urban was defined as ‘downtown core’ and ‘all other urban or suburban habitat, including quarries in an urban setting’, whereas in our study urban nests were only located in city cores. Gahbauer et al. (2015) also included coastal habitats within the definition of ‘rural’ habitats, which might provide increased prey densities from aggregations of coastal birds. Thus, Gahbauer et al.’s (2015) study included a much greater diversity of urban sites in North America, which may reflect the fact that peregrines have been urbanised in North America a lot longer than in Great Britain (Cade and Bird 1990). Another reason for the difference may be that rural and urban environments in Great Britain are likely to be very different to those in North America, for example, in terms of prey availability, prey diversity, and human population density.
Prey species diversity was lower in urban environments, supporting findings from other studies that bird diversity tends to decrease with urbanisation (e.g. Blair 1996; Clergeau et al. 1998; McKinney 2006; Tratalos et al. 2007), but this did not prevent peregrines from gaining a higher breeding success than rural peregrines. This is somewhat surprising because peregrines are generalist, opportunistic predators of most avian prey species (Ratcliffe 2010), and thus we would have expected that overall lower diversity could have influenced peregrine breeding success in urban centres negatively. In urban environments, however, the diet of urban peregrines typically consists of relatively few species such as pigeons and doves (Columbidae) and thrush species (Turdidae), that occur in very large numbers (Drewitt and Dixon 2008). These species have successfully colonised urban environments globally (e.g. Clergeau et al. 1998; Tratalos et al. 2007; Evans et al. 2010), and feral pigeons in particular tend to congregate around food sources in urban areas (Johnston and Janiga 1995), making them easier prey to target. The likely explanation for the lack of an effect of diversity, therefore, is that peregrines can afford to be specialists in urban environments. We were unable to test whether better predictions of reproductive success could be obtained by restricting prey availability to specific taxa. Nevertheless, although we expect that such analyses may improve the overall fit, the main effect of higher prey abundance for most prey species in urban environments will not change the main conclusion that abundance, rather than diversity, drives the success of urban peregrines.
Our results provide new evidence that urban environments may offer better habitat for some apex predators because of higher prey availability over a large spatial scale. It has been suggested that unless animals are followed throughout their lifetimes, not accounting for potential negative effects of urbanisation, for example greater exposure to disease (Krone et al. 2005; but see Suri et al. 2017), higher vehicle and building collision rates (Hager 2009), and poor food quality (Liker et al. 2008; Heiss et al. 2012; Meillère et al. 2015), may exaggerate the quality of urban environments for some species estimated from snapshot measures at single life history stages. Our estimates of quality were from the breeding season alone, and did not look at post fledgling survival, recruitment, or lifetime reproductive success, so we cannot say definitively that urban environments are better for peregrines in Great Britain. Furthermore, although the link we report with food availability in urban environments is correlational, it is based on model estimates rather than raw data, and the sampling period overlapped with a small proportion of the breeding data time series. Thus, it remains possible that the increased breeding performance may be due to other factors, for example increased temperatures or reduced persecution in urban environments (Chace and Walsh 2006), neither of which were investigated here. One reason for this high success may be a reduced threat of persecution in urban environments (Chace and Walsh 2006), which usually leads to complete failure of nesting attempts (Amar et al. 2012). However, we suspect persecution was relatively low at our rural sites as we did not include pairs nesting in habitats strongly associated with persecution, such as moorland managed for grouse shooting (Amar et al. 2012). Furthermore, the difference was detectable even when only successful pairs were included in our analyses, thus controlling for persecution. Finally, it might be expected that micro-climate differences between urban and rural sites could also be driving some of the differences in breeding performance, which remains to be tested, but including county in our analysis at least controlled for the possibility of systematic bias between geographic climatic variation and the distribution of urban and rural site.
In line with findings on other predators (e.g. Bloom and McCrary 1996; Parker 1996), our results provide evidence that the novel environments humans impose on wildlife may provide valuable habitats for some predators. They also suggest that prey availability is the key, which poses a difficult challenge for urban management because often important prey species are considered as pests in urban environments (Belant 1997; Sorace 2002). However, our results are limited for a number of reasons discussed above and not necessarily representative of all urban areas. Thus there is a clear need for future studies to focus on obtaining raw prey data at the same time as other key life history measures across individuals’ lives, and across as broad a gradient of urbanisation as possible. Only then will it be possible to determine whether urban environments are sources, sinks or self-sustaining habitats for apex predators (Marzluff et al. 2001; Battin 2004; Heard et al. 2012).
References
Altwegg R, Jenkins A, Abadi F (2014) Nestboxes and immigration drive the growth of an urban peregrine falcon Falco peregrinus population. Ibis 156(1):107–115
Amar A, Court IR, Davison M, Downing S, Grimshaw T, Pickford T, Raw D (2012) Linking nest histories, remotely sensed land use data and wildlife crime records to explore the impact of grouse moor management on peregrine falcon populations. Biol Conserv 145(1):86–94
Baker PJ, Ansell RJ, Dodds PAA, Webber CE, Harris S (2003) Factors affecting the distribution of small mammals in an urban area. Mammal Rev 33(1):95–100
Balmer D, Gillings S, Caffrey B, Swann B, Downie I, Fuller R (2013) BirdAtlas 2007 – 2011: the breeding and wintering birds of Britain and Ireland. BTO Books, Thetford
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool 287(1):1–23
Battin J (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18(6):1482–1491
Belant JL (1997) Gulls in urban environments: landscape-level management to reduce conflict. Landsc Urban Plan 38(3-4):245–258
Bishop CA, Brogan JM (2013) Estimates of avian mortality attributed to vehicle collisions in Canada. Avian Conserv and Ecol 8(2):2
Blair RB (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6(2):506–519
Bloom PH, McCrary MD (1996) The urban Buteo: red-shouldered hawks in southern California. In: Bird DM, Varland DE, Negro JJ (eds) Raptors in human landscapes: adaptations to built and cultivated environments. Academic Press, California, pp 31–39
Boal CW, Mannan RW (1999) Comparative breeding ecology of Cooper’s hawks in urban and exurban areas of southeastern Arizona. J Wildl Manag 63(1):77–84
Bonnington C, Gaston KJ, Evans KL (2015) Ecological traps and behavioural adjustments of urban songbirds to fine-scale spatial variation in predator activity. Anim Conserv 18(6):529–538
Brand AB, Snodgrass JW (2010) Value of artificial habitats for amphibian reproduction in altered landscapes. Conserv Biol 24(1):295–301
Burke, B.J., Clarke, D., Fitzpatrick, A., Carnus, T. and McMahon, B.J. (2015) Population status and factors affecting the productivity of peregrine falcon Falco peregrinus in County Wicklow, Ireland, 2008 – 2012. Biol Environ 115 (2): 115 – 124.
Cade TJ, Bird DM (1990) Peregrine falcons (Falco peregrinus) nesting in an urban environment: a review. Can Field Nat 104(2):209–218
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban 74(1):46–69
Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151(1):1–18
Charter M, Izhaki I, Bouskila A, Leshem Y (2007) Breeding success of the Eurasian kestrel (Falco tinnunculus) nesting on buildings in Israel. J Raptor Res 41(2):139–143
Clergeau P, Savard JL, Mennechez G, Falardeau G (1998) Bird abundance and diversity along an urban-rural gradient: a comparative study between two cities on different continents. The Condor 100(3):413–425
Conway CJ, Garcia V, Smith MD, Ellis LA, Whitney JL (2006) Comparative demography of burrowing owls in agricultural and urban landscapes in southeastern Washington. J Field Ornithol 77(3):280–290
Crick HQP, Ratcliffe DA (1995) The peregrine falcon Falco peregirnus breeding population of the United Kingdom in 1991. Bird Study 42(1):1–19
Demeyrier V, Lambrechts MM, Perret P, Grégoire A (2016) Experimental demonstration of an ecological trap for a wild bird in a human-transformed environment. Anim Behavi 118:181–190
Dhondt AA, Dhondt KV, Hawley DM, Jennelle CS (2007) Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol 36(3):205–208
Drewitt EJA, Dixon N (2008) Diet and prey selection of urban-dwelling peregrine falcons in southwest England. British Birds 101:58–67
Erritzoe J, Mazgajski TD, Rejt Ł (2003) Bird casualties on roads: a review. Acta Ornithologica 38(2):77–93
Evans KL, Newson SE, Gaston KJ (2009) Habitat influences on urban avian assemblages. Ibis 151(1):19–39
Evans KL, Hatchwell BJ, Parnell M, Gaston KJ (2010) A conceptual framework for the colonisation of urban areas: the blackbird Turdus merula as a case study. Biol Rev 85(3):643–667
Evans KL, Chamberlain DE, Hatchwell BJ, Gregory RD, Gaston KJ (2011) What makes an urban bird? Glob Chang Biol 17(1):32–44
Fischer JD, Cleeton SH, Lyons TP, Miller JR (2012) Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. BioScience 62(9):809–818
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Lett 3(4):368–370
Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ (2008) Garden bird feeding predicts the structure of avian urban landscapes. Divers Distrib 14(1):131–137
Gahbauer MA, Bird DM, Clark KE, French T, Brauning DW, McMorris FA (2015) Productivity, mortality, and management of urban peregrine falcons in northeastern North America. J Wildl Manag 79(1):10–19
Gaston KJ (2010) Urbanisation. In: Gaston KJ (ed) Urban ecology. Cambridge University Press, Cambridge, pp 10–34
Hager SB (2009) Human-related threats to urban raptors. J Raptor Res 43:210–226
Harris, S.J., Massimino, D., Newson, S.E., Eaton, M.A., Marchant, M.A., Balmer, D.E., Noble, D.G., Gillings, S., Procter, D. amd Pearce-Higgins, J.W. (2016) The Breeding Bird Survey 2015. BTO Research Report 687. British Trust for Ornithology, Thetford.
Heard G, Scroggie MP, Malone BS (2012) Classical metapopulation theory as a useful paradigm for the conservation of an endangered amphibian. Biol Conserv 148(1):156–166
Heesen M, Rogahn S, Ostner J, Schülke O (2013) Food abundance affects energy intake and reproduction in frugivorous female Assamese macaques. Behav Ecol Sociobiol 67(7):1053–1066
Heiss RS, Clark AB, McGowan KJ (2012) Growth and nutritional state of American crow nestlings vary between urban and rural habitats. Ecol Appl 19(4):829–839
Johnston RF, Janiga M (1995) Feral pigeons. Oxford University Press, Oxford
Jones DN, Reynolds J (2008) Feeding birds in our towns and cities: a global research opportunity. J Avian Biol 39(3):265–271
Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M (2010) Artificial night lighting affects dawn song, extra-pair siring success and lay date in songbirds. Curr Biol 20(19):1735–1739
Kettel EF, Gentle LK, Quinn JL, Yarnell RW (2018) The breeding performance of raptors in urban landscapes: a review and meta-analysis. J Ornithol 159(1):1–18
Korpimäki E, Norrdahl K, Rinta-Jaskari T (1991) Responses of stoats and least weasels to fluctuating food abundances: is the low phase of the vole cycle due to mustelid predation? Oecologia 88(4):552–561
Krone O, Altenkamp R, Kenntner N (2005) Prevalence of Trichomonas gallinae in northern goshawks from the Berlin area of northeastern Germany. J Wildl Dis 41:304–309
Liker A, Papp Z, Bókony V, Lendvai AZ (2008) Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J Anim Ecol 77(4):789–795
Lin W, Lin A, Lin J, Wang Y, Tseng H (2015) Breeding performance of crested goshawk Accipiter trivirgatus in urban and rural environments of Taiwan. Bird Study 62(2):177–184
Liven-Schulman I, Leshem Y, Alon D, Yom-Tov Y (2004) Causes of population declines of the lesser kestrel Falco naumanni in Israel. Ibis 146(1):145–152
Maciusik B, Lenda M, Skórka P (2010) Corridors, local food resources, and climatic conditions affect the utilization of the urban environments by the black-headed gull Larzus ridibundus in winter. Ecol Res 25(2):263–272
Martin TE (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Evol Syst 18:453–487
Marzluff JM (2001) Worldwide urbanisation ant its effects on birds. In: Marzluff JM, Bowman R, Donnelly R (eds) Avian ecology and conservation in an urbanising world. Springer Science, New York, pp 19–47
Marzluff JM, Bowman R, Donnelly R (2001) A historical perspective on urban bird research: trends, terms, and approaches. In: Marzluff JM, Bowman R, Donnelly R (eds) Avian ecology and conservation in an urbanizing world. Springer, Boston, pp 1–17
Massimino D, Johnston A, Noble DG, Pearce-Higgins JW (2015) Multi-species spatially-explicit indicators reveal spatially structured trends in bird communities. Ecol Indic 58:277–285
McKinney ML (2006) Urbanisation as a major cause of biotic homogenisation. Biol Conserv 127(3):247–260
Mearns R (1985) The hunting ranges of two female peregrines towards the end of the breeding season. J Raptor Res 19(1):20–26
Meillère A, Brischoux F, Parenteau C, Angelier F (2015) Influence of urbanisation on body size, condition, and physiology in an urban exploiter: a multi-component approach. PLoS ONE 10(8):e0135685
Mennechez G, Clergeau P (2006) Effect of urbanisation on habitat generalists: starlings not so flexible? Acta Oecologica 30(2):182–191
Newhouse MJ, Marra PP, Johnson LS (2008) Reproductive success of house wrens in suburban and rural landscapes. Wilson J Ornithol 120(1):99–104
Ordeñana MA, Crooks KR, Boydston EE, Fisher RN, Lyren LM, Siudyla S, Haas CD, Harris S, Hathaway SA, Turschak GM, Miles AK, Van Vuren DH (2010) Effects of urbanization on carnivore species distribution and richness. J Mammal 91(6):1322–1331
Orros ME, Thomas RL, Holloway GJ, Fellowes MDE (2015) Supplementary feeding of wild birds indirectly affects ground beetle populations in suburban gardens. Urban Ecosystems 18(2):465–475
Parker JW (1996) Urban ecology of the Mississippi kite. In: Bird DM, Varland DE, Negro JJ (eds) Raptors in human landscapes: adaptations to built and cultivated environments. Academic Press, California, pp 45–52
Peach W, Vincent K, Fowler JA, Grice PV (2008) Reproductive success of house sparrows along an urban gradient. Anim Conserv 11(6):493–503
Perrig M, Grüebler M, Keil H, Naef-Daenzer B (2014) Experimental food supplementation affects the physical development, behaviour and survival of little owl Athene noctua nestlings. Ibis 156(4):755–767
Pettett CE, Moorhouse TP, Johnson PJ, MacDonald DW (2017) Factors affecting hedgehog (Erinaceus europaeus) attraction to rural villages in arable landscapes. Eur J Wildl Res 63:54
Pollock CJ, Capilla-Lasheras P, McGill RAR, Helm B, Dominoni DM (2017) Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyanistes caeruleus). Sci Rep 7:5014
Ratcliffe DA (2010) The peregrine falcon, 2nd edn. T & AD Poyser Ltd., London
Rautio A, Isomursu M, Valtonen A, Hirvelä-Koski V, Kunnasranta M (2016) Mortality, disease and diet of European hedgehogs (Erinaceus europaeus) in an urban environment in Finland. Mammal Research 61(2):161–169
Rebolo-Ifrán N, Tella JL, Carrete M (2017) Urban conservation hotspots: predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci Rep 7:3527
Russo D, Ancillotto L (2015) Sensitivity of bats to urbanization: a review. Mammal Biol 80(3):205–212
Šálek M, Drahníková L, Tkadlec E (2014) Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mammal Rev 45(1):1–14
Schlesinger MD, Manley PN, Holyoak M (2008) Distinguishing stressors acting on land bird communities in an urbanising environment. Ecology 89(8):2302–2314
Shultz S, Bradbury RB, Evans KL, Gregory RD, Blackburn TM (2005) Brain size and resource specialization predict long-term population trends in British birds. Proc R Soc B 272(1578):2305–2311
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sims V, Evans KL, Newson SE, Tratalos JA, Gaston KJ (2008) Avian assemblage structure and domestic cat densities in urban environments. Divers Distrib 14(2):387–399
Snow DW, Perrins CM (1997) The birds of the western Palearctic. Oxford University Press, Oxford
Solonen T (2008) Larger broods in the northern goshawk Accipiter gentilis near urban areas in southern Finland. Ornis Fennica 85:118–125
Sorace A (2002) High density of bird and pest species in urban habitats and the role of predator abundance. Ornis Fennica 79:60–71
Sumasgutner P, Nemeth E, Tebb G, Krenn HW, Gamauf A (2014) Hard times in the city – attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front Zool 11:48. https://doi.org/10.1186/1742-9994-11-48
Suri J, Sumasgutner P, Hellard É, Koeslag A, Amar A (2017) Stability in prey abundance may buffer black sparrowhawk Accipiter melanoleucus from health impacts. Ibis 159(1):38–54
Tella JL, Hiraldo F, Donázar-Sancho JA, Negro JJ (1996) Costs and benefits of urban nesting in the lesser kestrel. In: Bird DM, Varland DE, Negro JJ (eds) Raptors in Human Landscapes: Adaptations to built and cultivated environments. Academic Press, California, pp 53–60
Tratalos J, Fuller RA, Evans KE, Davies RG, Newson SE, Greenwood JJD, Gaston KJ (2007) Bird densities are associated with household densities. Glob Chang Biol 13(8):1685–1695
Uttley JD, Walton P, Monaghan P, Austin G (1994) The effects of food abundance on breeding performance and adult time budgets of guillemots Uria aalge. Ibis 136 (2:205–213
Wauter LA, Lens L (1995) Effects of food availability and density on red squirrel (Sciurus vulgaris) reproduction. Ecology 76(8):2460–2469
Wilson MW, Balmer DE, Jones K, King VA, Raw D, Rollie CJ, Rooney E, Ruddock M, Smith GD, Stevenson A, Stirling-Aird PK, Wernham CV, Weston JM, Noble DG (2018) The breeding population of peregrine falcon Falco peregrinus in the United Kingdom, Isle of Man and Channel Islands in 2014. Bird Study 65(1):1–19
Acknowledgements
We thank all of the volunteers who monitored peregrine nests and provided breeding data. Special thanks go to Gloucestershire Raptor Monitoring Group (in particular G. Kirk, S. Watson and N. Wylde), Peak District Raptor Monitoring Group (in particular T. Grimshaw), Shropshire Peregrine Group (in particular J. Turner), London Peregrine Partnership, D. Pearce and G. Roberts. Thanks also go to the BTO for providing BBS data and to all of the volunteers who have carried out BBS surveys. The BBS is funded by a partnership of BTO, JNCC and RSPB. We also thank I. Barber for his comments on an earlier draft of the manuscript. This research was funded by a Nottingham Trent University Vice Chancellor’s bursary scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kettel, E.F., Gentle, L.K., Yarnell, R.W. et al. Breeding performance of an apex predator, the peregrine falcon, across urban and rural landscapes. Urban Ecosyst 22, 117–125 (2019). https://doi.org/10.1007/s11252-018-0799-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-018-0799-x