Abstract
Probiotics are non-pathogenic microorganisms that are potentially important non-antibiotic alternatives. This study aimed to compare novel multi-strain and single-strain Bacillus probiotics and their respective influences on broiler chickens’ performance, gut health, litter quality, immune response, and NBN and TLR gene expression. A total of 1200 Arbor-Acres 1-day-old broiler chicks were randomly allocated into three treatments (T1 was a control, T2 was supplemented with a combined Bacillus coagulans (2 × 109 cfu/g) and Bacillus licheniformis (8 × 109 cfu/g) probiotic strains (0.2 kg/ton of feed), and T3 was supplemented with Bacillus licheniformis (3.2 × 109 cfu/g) probiotic (0.5 kg/ton of feed) with eight replicas of each. Supplementing the broiler diet with either the single-strain (T3) or the multi-strain (T2) Bacillus-based probiotic raised the overall birds’ body weight, body weight gain, feed conversion ratio, and European production efficiency factor compared to the control (T1), with a significant enhancement achieved by the multi-strain Bacillus product (P = 0.005). T2 and T3 exhibited significantly improved cholesterol, Alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and alkaline phosphatase levels than the control (P ≤ 0.05). The transcript levels of both NBN and TLR genes were upregulated in the liver in the T2 and T3 groups. The T2 group experienced significant reductions in gut bacterial counts, especially for Clostridia, and recorded the lowest litter moisture and nitrogen. In conclusion, supplementing broiler diets with probiotics of multiple Bacillus strains increased production profitability by promoting bird growth, improving feed intake, enhancing gut mucosa and immune organs, and upregulating genes responsible for immunity. All these inhibit the overgrowth of enteric pathogens and sustain litter quality.
Similar content being viewed by others
Introduction
In the previous 50 years, poultry producers integrated antibiotics as growth promoters into broiler diets at sub-therapeutic doses to control the overgrowth of gastrointestinal disease-causing microorganisms, predominantly Clostridium perfringens and coccidiosis (Mehdi et al., 2018). However, the frequent use of antimicrobials was implicated in the progression of bacterial resistance, which is a public health problem. Hence, the use of antibiotics as growth promoters has been banned by the European Union (ESVAC, 2017). Accordingly, the poultry feed industry sought out sustainable non-antibiotic alternatives to maintain a high production performance while protecting the environment and human health. Additionally, consumers are showing a growing interest in organic poultry meat (Hafez and Shehata, 2021).
Probiotics are beneficial live bacteria when supplemented in broiler feed can promote growth, intestinal health, and bird immunity to infections (Gernat et al., 2021; Tellez-Isaias et al., 2021). In addition, probiotics could modulate lipid metabolism and reduce serum cholesterol levels, through bile salts’ deconjugation with bile salt hydrolase enzyme (Reis et al., 2017; Hussien et al., 2022). The Bacillus bacteria are considered among the best probiotics discovered because of their immense attributes of releasing antimicrobial substances effective on pathogenic microorganisms, as well as their capacity to survive in harsh environments (Hong et al., 2005). Previous research studies focused on some bacilli probiotic strains, such as B. subtilis, B. coagulans, B. licheniformis, B. cereus, and B. polymyxa (Amoah et al., 2021).
Bacillus coagulans and Bacillus licheniformis are Gram-positive, spore-formers bacteria, that are commonly used as commercial probiotics for poultry feed (Moeller et al., 2009; Cutting, 2011). Due to their protective protein coat and other mechanisms, they can resist environmental stressors during the pelleting process, storage, and handling (Upadhaya et al., 2019a, b; Bonos et al., 2021), as well as survive stomach acidity and pass to the intestine where it germinates and grows safely without encoding for enterotoxins (Endres et al., 2009; Xing et al., 2021). B. coagulans is a homofermentative strain that utilizes hexose efficiently and has been recently considered a novel and safe probiotic. B. coagulans produces L-lactic acid as the primary byproduct of glucose fermentation (approximately 97% of the fermented products) while producing acetate and succinate as minor products (Qin et al., 2009; Endres et al., 2009). B. coagulans maintain the intestinal mucosal barrier by improving the intestinal flora, promoting the regeneration of broilers’ intestinal epithelial cells, and enhancing innate immunity (Liu et al., 2022). B. licheniformis have been extensively used in the poultry industry as a growth promoter to improve bird growth. Additionally, B. licheniformis was used as a prebiotic that combat Clostridia intestinal colonization in commercial broiler production (Zhou et al., 2016). Dietary supplementation of broiler diet with B. licheniformis could facilitate nutrient degradation and absorption by their ability to produce protease, lipase, and amylase enzymes and therefore increase feed efficiency and improve growth (Rozs et al., 2001).
To date, there are few published studies conducted on broiler chickens to examine the combined dietary Bacillus coagulans and Bacillus licheniformis probiotic supplementation to broiler chickens on birds’ performance, gut health, and immune responses. Consequently, the present research aims to evaluate and compare the efficacies of two products of bacilli-based probiotics on broiler’s growth performance, carcass traits, blood biochemistry, gut health, genes expression, and cecal bacteria, in addition to litter chemical and microbial qualities. The first product was a novel multi-strain Bacillus probiotic composed of both Bacillus coagulans (DSM 32,016; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g), while the second probiotic product was from the commercial market and consisted of a single-strain, Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g).
Materials and methods
Ethics approval
The current experiment was accepted according to the Institutional Animal Care and Use Committee guidelines, Faculty of Veterinary Medicine, Cairo University, Egypt. (Ethical reference No: Vet CU28/04/2021/280).
Bird husbandry
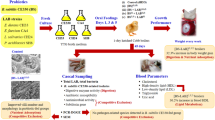
The experimental study was performed at the Animal and Poultry Research Center, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. A total of 1200 Arbor-Acres 1-day-old, unsexed broiler chicks were obtained from a commercial hatchery, weighed (average initial weight 44.5 gm), and randomly allocated to three treatments (groups) in a completely randomized design, each treatment containing 8 replicates (pens) with 50 birds/replicate (stocking density was 10 birds/m2). Birds of different replicates were housed in separate floor pens (2.9 × 1.7 m) with a 7–10 cm height of clean wood shavings litter in a naturally ventilated open house for 35 days. The environmental temperature in the first 3 days of life was 33 °C, and it gradually decreased by 2.8 °C each week until it reached 24 °C and remained constant for the remainder of the experiment. The relative humidity was maintained at about 55–60% throughout the entire period of the trial. Birds were kept under 24-h lighting for the first 3 days and then maintained under 23 L:1 D for the remainder of the study. The vaccination schedule was as follows: on day 6, the birds were vaccinated against the Newcastle disease virus (NDV-Hitchner B1) through eye dropping. On day 14, the birds got infectious bursal disease (IBD) and avian influenza (H5N1) vaccines via S/C injections (0.2 ml/bird) and on day 18, they received the NDV-Lasota vaccine through eye droppings.
Experimental design
The birds were fed a corn-soya bean meal-based diet that was formulated to meet the nutrient requirements for broilers according to the Arbor-Acres Broiler Nutrition Supplement (Aviagen, 2009), while feed and water were provided ad libitum. The birds were fed a starter crumble diet (1–14 days), which was changed to a grower mash diet instead of normal grower pellet form (15–27 days), and finally finisher pelleted diet (28–35 days) (Table 1). The birds were equally and randomly divided into three treatment groups. The birds in the first treatment group, which was considered the control group (T1), were only fed the basal diet (Table 1). The birds in the second treatment group (T2) were fed a basal diet supplemented with a multi-strain probiotic formula of naturally occurring strains of lactic acid producer Bacillus coagulans (DSM 32,016; 2 × 109 cfu/g) and pathogen inhibitory Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g) provided on a calcium carbonate carrier at a rate of 0.2 kg/ton of feed (TechnoCare®, BIOCHEM Zusatzstoffe Handels-und Produktionsgesellschaft mbH, Lohne, Germany). The birds in the third treatment group (T3) were fed a basal diet supplemented with a commercial single-strain probiotic product from the commercial market based on a naturally occurring strain of Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g) provided on a calcium carbonate carrier at a rate of 0.5 kg/ton of feed (GalliPro® Tect, Chr. Hansen Holding A/S, Hørsholm, Denmark). This strain was selected specifically for its ability to germinate and multiply fast in the gut and its ability to interact with other microorganisms.
Growth performance
Body weights were recorded at arrival and once every week until 35 days of age (20 birds/group). The weekly body weight gains (BWG) and feed intakes (FI) were determined by calculating the changes between the primary and final weight, and between the offered and remaining quantity of feed, respectively. The FI/week was then divided by the total number of birds in each replicate after adjusting for mortality to get the average weekly FI/bird. Feed conversion ratio (FCR) was measured by dividing the weekly FI by the weekly BWG considering the dead birds. The European Production Efficiency Factor (EPEF) was calculated (Marcu et al., 2013) as follows:
Throughout the experiment, the daily mortality was documented for every replicate and the mortality rate was calculated.
Carcass traits and immune organs’ relative weights
At the age of 35 days, eight birds from each treatment were randomly selected (close to the average weight), weighed, and slaughtered after 12 h of fasting for complete gut evacuation. After removing the head, neck, and legs, each bird was scalded, de-feathered, and eviscerated, and the carcass was weighed and expressed as a percentage of its live weight (carcass yield). The carcass was dissected, and the relative weights of the breast, drumstick, and thigh were measured. In addition, the weights of the gizzard, liver (without gall bladder), heart, spleen, and bursa of Fabricius were recorded and their percentages relative to the carcass weight (the relative organ weights) were calculated.
Sampling of litter and intestinal content
Litter sampling was carried out at 10, 23, and 35 days of age in each group (8 samples/group). The top 7 cm of deep litter were scooped from three different locations of each replicate pen and placed in sterile plastic bags (Dumas et al., 2011; Lopes et al., 2013). Cloacal swabs and cecal contents (eight samples per group) were collected from birds on days 23 and 35, respectively. All samples were stored at 4 °C till further bacteriological examination.
Litter physical and chemical examinations
The moisture content of litter was determined as previously reported by Dumas et al. (2011). Weighed litter (10 g) from each replicate was subjected to drying at 100 °C ± 5 °C for 24–48 h in a hot air oven. The moisture content percentage was determined by subtracting dry litter weight from the initial weight. Determination of the total nitrogen content of the litter was calculated as total Kjeldahl nitrogen (Jackson, 1973).
Microbiological examinations of litter and intestinal content
For microbial examinations of each litter sample, 3 g of homogenized litter were diluted in tubes containing 27 ml of sterile saline solution (10−1 dilution), left for 30–60 min at room temperature, and frequently shaken to allow the litter to mix well with the diluent (Dumas et al., 2011; Lopes et al., 2013). For microbial examinations of cecal contents, 1 g was diluted and homogenized in tubes containing 9 ml sterile saline solution (10−1 dilution) (Esmaeilipour et al., 2012). The samples were subjected to tenfold serial dilution in tubes containing 9 ml aliquots of sterile saline solution. From the serially diluted tubes, 100 µl samples were spread onto Nutrient Agar and Reinforced Clostridial Agar (Oxoid Ltd, Basingstoke, Hants, UK) to enumerate the total aerobes, total anaerobes, and Clostridia, respectively. Plates were then put in incubation at 37 °C for 24–48 h, and tightly sealed anaerobic jars were used to achieve anaerobic conditions. Finally, the counts of bacterial colonies for each 1 g of litters and caecal contents were reported as mean 10 logarithm colony forming units (log10CFU).
Confirmation and toxin typing of Clostridium perfringens
The genomic DNA of suspected Clostridium perfringens isolates was extracted by means of an extraction kit (QIAamp mini kit, Qiagen, Hilden, Germany). The multiplex PCR assay was employed to identify the presence of genes encoding alpha-toxin (cpa), beta-toxin (cpb), epsilon-toxin (etx), iota toxin (iap), in addition to enterotoxin gene (cpe). Primer sequences were published previously (Ghoneim and Hamza 2017).
The multiplex PCR assay was conducted per the method described by Ghoneim and Hamza (2017). The PCR reaction mixtures were analyzed by electrophoresis on a 1.5% (w/v) agarose gel in the presence of a 100 bp DNA ladder (Fermentas Life Science, USA).
Blood biochemical analysis
On day 35, blood samples (eight samples/group) were randomly collected for separation of serum by centrifugation at 5000 rpm for 15 min, and then stored at − 20 °C for further analysis. The serum samples were analyzed spectrophotometrically (UV-2100 Spectrophotometer, USA) for total cholesterol (at 500 nm wavelength), triglycerides (505 nm), total protein (546 nm), albumin (578 nm), globulin (calculated from the equation of total protein–albumin), albumin globulin ratio (A/G), alanine transaminase (ALT) (340 nm), aspartate transaminase (AST) (340 nm), lactate dehydrogenase (LDH) (340 nm), alkaline phosphatase (ALP) (405 nm), and uric acid (546 nm) using reagent kits of Spectrum Diagnostics Egyptian Company for Biotechnology, Egypt.
Evaluation of the ND vaccinal immune status
As recommended by the OIE (2022), the hemagglutination inhibition (HI) test was used for evaluating the vaccinal immune status of the birds (8 samples/group). Two-fold dilutions were performed for 25 µl volumes of serum samples in 99-V-bottomed microwell plastic plates and tested against 25 µl volumes of four hemagglutination units (4 HAU) of the ND virus commercial antigen (Lasota). Plates were incubated at room temperature for 20 min, then 25 µl aliquots of a 1% suspension of packed chicken RBCs were added to each well. Antibody titers were reported as mean log2HI titers.
Histological examination
On day 35, the liver, intestine, and immune organs “spleen and bursa of Fabricius” (eight samples of each organ/group) were dissected from bird carcasses. Sections from the middle of the duodenum, jejunum, ileum (all measuring about 0.5 cm in length), and cecum were removed, opened lengthwise, and gently flushed with 0.1 M phosphate-buffered salines (pH 7.4) then immediately preserved in 10% neutral buffered formalin solution. To investigate the histological structure, 6–7 thick sections were stained with Delafield’s iron haematoxylin and eosin according to Bancroft and Gamble (2008).
Histomorphometric investigation of the intestine, spleen, and bursa of Fabricius
Both the depth of the intestinal gland and the villus length, excluding the intestinal crypt, were measured for histomorphometry.
The diameters of the cortical and medullary regions of the follicle of the spleen and bursa of Fabricius were measured (no.). At the Faculty of Veterinary Medicine, Cairo University, stained sections of each bird were evaluated using a Leica Quin 500 analyzer computer system (Leica Microsystems, Switzerland). To transform the measurement units (pixels) generated by the image analyzer program into real micrometer units, the image analyzer was automatically calibrated. For each sample, the photos of each segment were taken at a final magnification of 40 × .
Transcriptional expression of growth, stress, DNA repair, and immune response-related genes in tissue samples
Real-time PCR (qRT-PCR) was used to determine the expression of mTOR (growth-related gene) in breast and thigh muscles, as well as NBN (stress and DNA repair-related gene) and TLR4 (immune response-related gene) in the liver in the broiler chicken (8 birds/group). Total RNA was extracted from the different tissue samples (eight samples of each tissue per group) by an easy-spin Total RNA Extraction Kit (iNtRON Biotechnology DR, Cat. No.17221). The concentration and purity of RNA were evaluated using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies) (Kasas et al., 2022). The cDNA synthesis was done by M-MuLV Reverse Transcriptase (NEB#M0253) and the expression levels of mRNA were determined by qRT-PCR using HERAPLUS SYBR Green qPCR kit (#: WF10308002). The using primers were designed by the Primer 3 program (https://primer3.ut.ee) and their sequences are presented in Table 2. (Hassan et al., 2022). Each RT-PCR was carried out 3 times (Kamel et al., 2018). The β-actin gene was used as the reference gene. The obtained data were analyzed using CT, ΔCT, ΔΔCT, and 2− ΔΔCT (Livak and Schmittgen, 2001).
Statistical analysis
PASW Statistics, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Results were reported as means and standard errors of means (SEM). One-way ANOVA was applied for comparing the mean values of control and test groups, while post hoc comparisons were determined using Tukey’s test. The threshold of P ≤ 0.05 was set for statistical significance. Boxplots were produced by R (R Foundation for Statistical Computing), version 3.6.1 (Team, 2019), based on ggplot2 (Wickham, 2016; Kassambara, 2019) packages.
Results
Performance parameters
Table 3 displays the weekly performance parameters of the birds. From day 21 onward, significant increases in body weights were observed in T2 and T3 birds (P < 0.05). On day 35, the highest values of body weight gain were recorded for T2 (+ 100 g/bird; + 14.7%), then T3 (+ 86 g/bird; + 12.6%), compared to the control (T1). At the end of the finisher phase (5th week), T2 displayed the lowest average feed consumption, with − 45 g feed/bird (− 3.6%), which was lower than the control (T1) and − 75 g feed/bird (− 5.8%) compared to T3 (P < 0.001). On average, the FCR in the 5th week recorded 1.56 g/g (− 16.6%) for T2 and 1.68 g/g (− 10.2%) for T3 compared to 1.87 g/g for the control, with a statistically significant difference of (P = 0.012). T2 exhibited the most efficient performance parameters at the grower and finisher phases.
Table 4 presents the cumulative performance along the 35 days. T2 and T3 showed significantly better body weights, body weight gains, and FCR (P = 0.001, 0.001, and 0.0001 respectively) than the control (T1). Feed intake was more efficient in T2 than in T1 and T3 (− 45 and − 75 g feed/bird, respectively) (P = 0.014). No significant differences in survival and mortality rates were recorded between treatments (P > 0.05). Accordingly, the calculated EPEF revealed a significantly elevated value for T2 when compared to the control (P = 0.005).
Carcass traits and immune organs’ relative weights
In Table 5, T2 and T3 showed an 8.5% increase in the breast weight percentage compared to the control. The bursa weight percentage recorded 26% and 14% increases in T2 and T3, respectively, compared to the control. However, no significant differences in carcass traits (dressing yield, breast, thigh, drum, liver, and gizzard relative weights) or immune organs’ weight percentages were revealed between treatments and control (P > 0.05).
Bacterial counts of cloacal swabs and cecal content
Figure 1 presents the aerobic, anaerobic, and clostridial bacterial counts of cloacal swabs (day 23) and cecal contents (day 35). From day 23 to day 35, the overall bacterial counts exhibited a 4.72–5.15 log10 CFU increase. On day 23, cloacal swabs of T2 recorded the lowest aerobic, anaerobic, and clostridial bacterial counts (means = 5.33, 5.23, and 5.10 log10 CFU, respectively), with significant differences with the control (T1) (means = 5.48, 5.53, and 6.75 log10 CFU, respectively) and T3 (means = 5.83, 5.70, and 6.05 log10 CFU, respectively) (P = 0.014). On day 35, T2 birds displayed the lowest cecal aerobic, anaerobic, and clostridial bacterial counts (means = 10.28, 9.50, and 9.90 log10 CFU/g, respectively), and these values differed significantly from those of the control (means = 10.40, 11.10, and 10.73 log10 CFU/g, respectively), and T3 (means = 11.13, 11.30, and 11.45 log10 CFU/g, respectively). Unfortunately, T3 did not show efficiency in lowering cecal microbial counts (especially clostridial counts) at any age compared to the control (T1).
Influence of dietary Bacillus strains probiotic supplements on cloacal (day 23) and cecal (day 35) bacterial counts of broiler chickens (n = 8 birds/group); T1, control group (basal diet); T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed; T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 10.9 cfu/g))/ton of feed; significance was indicated at P ≤ 0.05
Litter chemical and microbiological qualities
Table 6 presents data on litter quality. On day 35, litter nitrogen was significantly reduced in T2 and T3 compared to the control T1, with T2 recording the lowest significance level (P = 0.0001). On the other hand, the peak of litter moisture was recorded on day 23 in all groups after feeding the birds the mash grower diet. On day 35, T2 showed the lowest litter moisture while T3 showed the highest level; however, the differences were not statistically significant (P > 0.05). Bacteriological examination of litter revealed relatively higher bacterial counts in T1 compared to T2 and T3. However, T3 showed elevated litter bacterial counts on day 35, primarily for Clostridia.
Confirmation and toxin typing of Clostridium perfringens
The multiplex PCR of suspected C. perfringens isolates revealed that the amplification of the alpha-toxin gene at 324 bp represented C. perfringens type A.
Blood biochemical parameters
Serum biochemistry results are presented in Table 7. T2 showed an 11% increase in serum albumin and a 6.3% reduction in uric acid compared to the control. However, no significant differences were found between the groups in the uric acid or protein profiles of sera (total protein, albumin, globulin, and A\G ratio). The cholesterol concentration significantly decreased in T3 (P = 0.001). AST, ALT, LDH, and ALP activities were significantly reduced in T2 and T3 compared to T1 (P < 0.05).
ND vaccinal HI titers
The results of HI titers against NDV are displayed in Fig. 2. T2 and T3 exhibited lower HI titers (3.17 ± 0.65 and 4.25 ± 0.48 log2, respectively) compared to T1 (6.40 ± 0.81 log2); (P = 0.015).
Influence of dietary Bacillus strains probiotic supplements on serological mean HI titers (log2) toward NDV vaccination of broiler chickens (*n = 8 birds/group). Data are represented as mean ± SEM. a,b Bars showing different superscripts point to significant differences (P ≤ 0.05). T1, control group (basal diet); T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed; T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g))/ton of feed
Intestinal and immune organ histology and histomorphometry
Histology of the liver, small intestine, and large intestine
The histological findings of the liver are presented in Fig. 3. The histological structure of the liver parenchyma was normal in T1, as the hepatocytes were radiated from the central vein in cords in between the blood sinusoids (Fig. 3A). The portal area contained the bile duct, hepatic artery, and portal vein with normal lymphocytic infiltration (Fig. 3B). Lymphocytic and eosinophilic leukocytic aggregations were normally found around the vessels and between the cords (Fig. 3C). T2 showed a severe increase in the lymphocytic infiltration within the portal area compared with the control (Fig. 3D). Moreover, the lymphocytic and eosinophilic leukocytic aggregations were increased around the vessels and between the cords (Fig. 3E). Additionally, a mild increase in the lymphocytic infiltration was observed within the portal area in liver tissues of T3 compared with those of T2 (Fig. 3F).
Liver sections of chickens. (A–C) Control group (T1) demonstrating A normal hepatic parenchyma, hepatic cord (yellow arrow) radiating from the central vein (CV), and blood sinusoids (red arrow). H&E. X400 B Portal area containing bile duct (BD), hepatic artery (HA), and portal vein (PV) with normal lymphocytic infiltration (arrows). H&E. X400 C Lymphocytic and eosinophilic leukocytic aggregations (arrow) around the vessels and between the cords. H&E. X400. (D, E) T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed showing (D) sever increase in the lymphocytic infiltration (arrows) within the portal area. Notice: bile duct (BD), hepatic artery (HA), and portal vein (PV). H&E. X400. E Increase lymphocytic and eosinophilic leukocytic aggregations (arrows) around the vessels and between the cords. H&E. X100. F T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 10.9 cfu/g))/ton of feed showing mild increase in the lymphocytic infiltration (arrow) within the portal area. Notice: bile duct (BD), hepatic artery (HA), and portal vein (PV). H&E. X400
The histological results of the sections of the small intestines are presented in Fig. 4. T2 showed the appearance of lymph nodules in the lamina propria of the duodenum and jejunum (Fig. 4A and B). Meanwhile, T3 showed an increase in the lymphoid tissue of the lamina propria of the duodenum only (Fig. 4C). Examination of large intestine sections of the control (T1) revealed a normal histological structure with short intestinal villi. The lamina propria contained lymphoid cells and lymph nodules with a normal thickness of the tunica muscularis (Fig. 4D). However, large intestine sections from birds of T2 showed a severe increase in the lymphoid tissue and lymph nodules of the lamina propria (Fig. 4E). Moreover, a noticeable increase in the thickness of the tunica muscularis was observed compared with the control (Fig. 4F). A mild increase in the lymphoid tissue and lymph nodules of the lamina propria and the thickness of the tunica muscularis were also noticed within the large intestine tissues of T3 compared with T2 (Fig. 4G).
(A–C) Small intestine sections from poultry. A, B T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed showing appearance of lymph nodules (LN) in lamina propria. H&E. X100 A Duodenum B jejunum C T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g))/ton of feed group (T3) showing duodenum with increase in the lymphoid tissue of the lamina propria. H&E. X100. (D–G) Large intestine sections from poultry (D) control group (T1) showing short intestinal villi (arrow), lamina propria (LP) containing lymphoid cells and lymph nodules, and normal thickness of tunica muscularis (M). H&E. X100. E, F T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed showing (E) sever increase in the lymphoid tissue and lymph nodules (LN) of the lamina propria (LP). H&E. X100. F Increase in the thickness (arc) of the tunica muscularis (M). H&E. X100. G T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 10.9 cfu/g))/ton of feed showing mild increase in the lymphoid tissue and lymph nodules (LN) of the lamina propria (LP) and in the thickness (arc) of the tunica muscularis (M). H&E. X100
Histomorphometry of the small intestine, spleen, and bursa
The results in Table 8 present the histomorphometry of the small intestine. The length of the intestinal villi of the duodenum, jejunum, and ilium showed significant increases in T2 and T3 compared to T1. However, T2 and T3 had significantly reduced duodenal crypt depths compared to T1. Deeper crypts were recorded in T2’s jejunum and T3’s ileum (P = 0.0001).
The results in Fig. 5 introduced the histomorphometry of immune organs (spleen and bursa). T3 and T2 showed significantly higher diameters of the bursa (including the cortex and medulla) and the white pulp of the spleen (P < 0.0001) than those of the control. Eventually, T3 recorded the maximum immune organ measures compared to T2 and T1.
Influence of dietary Bacillus strains probiotics supplements on histomorphometry immune organs of broiler chickens (n = 8 birds/group). A Bursa and B spleen. Data are represented as mean µm and SEM. a,b,c Bars showing different superscripts point to significant differences (P ≤ 0.05). T1, control group (basal diet); T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed; T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g))/ton of feed
Gene expression
In Figs. 6 and 7, the mRNA relative expression levels of NBN, TLR, and mTOR tissue genes were plotted. In liver tissues of T2 and T3, the transcript levels of NBN (stress and DNA repair-related gene) and TLR (immune response-related gene) genes were significantly more upregulated than those in T1. The upregulation of TLR mRNA was the highest in T2 (Fig. 6). The transcript level of the mTOR gene (growth-related gene) was examined in both the breast and thigh muscles of broiler chickens. There were no significant differences in the expression level of the mTOR gene between the three groups (Fig. 7).
Influence of dietary Bacillus strains probiotics supplements on m-RNA relative expression levels of the different genes; NBN (stress and DNA repair-related gene) and TLR (immune response-related gene) in the liver tissue of broiler chickens (n = 8 birds/group). Data are represented as means and SEM. a,b,c and A,B,C Bars showing different superscripts point to significant differences (P ≤ 0.05). T1, control group (basal diet); T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed; T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g))/ton of feed
Influence of dietary supplementation of Bacillus strains probiotics on m-RNA relative expression levels for mTOR gene (growth-related gene) in both breast and thigh muscles of broiler chickens (n = 8 birds/group). Data are represented as means and SEM. Significant difference was set at P ≤ 0.05. T1, control group (basal diet); T2, basal diet + 0.2 kg multi strains probiotic (Bacillus coagulans (DSM 32,806; 2 × 109 cfu/g) and Bacillus licheniformis (DSM 33,806; 8 × 109 cfu/g))/ton of feed; T3, basal diet + 0.5 kg single strain probiotic (Bacillus licheniformis (DSM 17,236; 3.2 × 109 cfu/g))/ton of feed
Discussion
In this study, we explored the effects of adding Bacillus as a probiotic feed supplement to broiler chickens, using either a single strain product (Bacillus licheniformis) or a multi-strain formula of Bacillus coagulans and Bacillus licheniformis. Our results revealed that the two formulations of Bacillus probiotics enhanced the weekly and overall growth performance of the birds in T2 and T3, especially from day 21 onward. Bacilli probiotics were thought to express their growth-enhancing impacts at later growth stages (Upadhaya et al., 2019a, b; Ahmat et al., 2021). However, feeding birds the probiotic with two Bacillus strains (T2 = Bacillus coagulans + Bacillus licheniformis) achieved a better growth rate and more efficient feed intake than feeding them a single Bacillus probiotic (T3 = Bacillus licheniformis). This significant improvement could be due to the growth-promoting effect of Bacillus coagulans via the synthesis and metabolism of proteins, short-chain fatty acids, and vitamins (LeBlanc et al., 2017). Once activated and germinated, Bacillus coagulans may aid in digesting carbohydrates and proteins, helping the birds gain weight (Maathuis et al., 2010). Moreover, adding multiple strains of Bacillus to broiler rations significantly enhanced body weight gain and the FCR (Ahmat et al., 2021; Wealleans et al., 2017).
Although the grower feed was administered as mash instead of pellets, birds supplemented with both Bacillus coagulans and Bacillus licheniformis (T2) overcame that stressor and achieved an improved FCR. These findings could be attributed to the enhanced secretion of endogenous enzymes (such as amylase, protease, and lipase) stimulated by Bacillus coagulans, which boosts feed digestibility and availability, and intestinal peristalsis (Upadhaya et al., 2019a, b; LeBlanc et al., 2017; Gu et al., 2015). Furthermore, Bacillus coagulans may improve the feed conversion ratio of broiler chickens by promoting a healthy balance of intestinal microbiota (Hung et al., 2012). Bacilli-supplemented birds in T2 showed higher EPEF values than those in the control group, which indicated the improvement of economic benefits as stated by Zhang et al. (2021).
The results of the current study revealed that dietary probiotics of multiple strains (T2) increased the relative weight (%) of breast muscle compared to the control and T3, and similar findings were reported by Ahmat et al. (2021). Other carcass traits did not exhibit any increase due to the inclusion of Bacillus probiotics compared to the control, which was in line with the findings of other previous studies (Upadhaya et al., 2019a, b; Bahrampour et al., 2020; Upadhaya et al., 2019a, b). Dietary supplementation with B. coagulans and/or B. licheniformis increased bursa relative weights in T2 and T3 birds. The bursa of Fabricius is responsible for humoral immunity in birds. Bacillus-based probiotics have the ability to regulate broilers’ immune systems (Ahmat et al., 2021). Other studies had reported that bacilli-based probiotics had no effect on the weight of immune organs (spleen and bursa) (Ghahri et al., 2013; Yun et al., 2017). NDV antibody titers in T3 supplemented with 0.5 kg Bacillus licheniformis probiotic/ton were higher than in T2 supplemented with 0.2 kg Bacilli probiotic/ton. This variation could be attributed to the different dosages administered to the treatment groups (Ahmat et al., 2021) or strain-to-strain variability.
The results of serum biochemistry revealed that T2 expressed higher serum albumin concentrations than the control, even though the differences were not statistically significant. Zhu et al. (2020) reported that there were no significant differences in the plasma concentrations of total protein, albumin, and triglyceride because of heat-inactivated probiotics. Albumin, which is produced mainly by the liver, is a principal element of total protein. The increased amount of serum albumin in bacilli-supplemented birds was attributed to the antibacterial metabolites secreted by Bacillus that inhibit the growth of pathogens and enhance the utilization of dietary protein (Ahmat et al., 2021). Adding multi-Bacillus strains to T2 reduced serum uric acid levels compared to the control. Uric acid is the nitrogenous excretory product of protein metabolism in birds and the measurement of its serum levels is one of the renal function tests. This result indicated that bacilli probiotics reduced the pressure on birds’ kidneys by reducing the serum non-protein nitrogen as uric acid (Ahmat et al., 2021).
Serum cholesterol levels in T3 and T2 were lower than in the control group. Cholesterol is included in developing cell membranes, and bile formation, and is a precursor of vitamin D and many hormones. The hypocholesterolemic effect of both probiotics is attributed to their ability to bind cholesterol in the small intestine (Ahmat et al., 2021; Manafi et al., 2017). Serum TAG decreased due to the addition of the combined Bacillus coagulans and Bacillus licheniformis probiotic compared to the control; however, the decrease was not statistically significant as reported previously (Zhu et al., 2020). Zhou et al. (2016) reported that B. licheniformis can improve lipid metabolism in mice that were fed a high-fat diet.
Enhanced liver functions were identified in sera of T2 and T3 as reduced ALT and AST concentrations, which indicated the significant efficacy of bacilli in the protection of treated birds from hepatocellular damage compared to controls (Hussein et al., 2020). Also, bacilli probiotics lowered ALP and LDH activities in T2 and T3 sera. The ALP enzyme is considered a biomarker for other tissues such as bone and renal tissues. The decrease in ALP activity indicates the ameliorative effect of both probiotics on bone and kidney functions (Sharma et al., 2014; Rashidi et al., 2020). Additionally, LDH is recognized as a marker for cell injuries and tissue damage. The obtained results suggested a decline in LDH activity in the serum of broiler chickens that were fed bacilli probiotics indicating fewer cell injuries (Mazkour et al., 2020).
The expressions of the NBN, TLR4, and mTOR genes and their relationship with the body performance of broiler chickens were investigated. The NBN gene codes for the Nibrin protein, which is involved in many critical cellular functions, including DNA repair of damaged lesions by guiding repair proteins to sites of DNA damage, which in turn prevents uncontrolled cell division and/or death (Khalaf et al., 2020). T2 and T3 expressed significantly higher levels of NBN compared to T1. This could be because the change in the physical form of the grower diet to mash instead of pellets may cause stress on broiler chickens. Stress may increase damage to biomolecules, including DNA (Czarny et al., 2018). Stress-induced DNA damage might stimulate the expression of the NBN gene to provide genomic stability and DNA repair which appeared in the groups that were fed Bacillus probiotics. Moreover, a significant increase in TLR4 gene expression in T2 and T3 was observed. TLRs are evolutionarily conserved membrane receptors widely expressed on various innate immunity cells and non-immune cells for sensing abnormal factors, stimulating the innate immune system’s response (Rozs et al., 2001). Stress has been associated with changes in immune parameters, including an increase in innate immunity. TLRs appear as the first line of defense against invading environmental stressors, and their expression is modulated (Gárate et al., 2013). Gárate et al. (2013) reported that stress exposure upregulated the TLR-4 pathway in the prefrontal cortex of mice. On the other hand, the transcript level of the mTOR gene did not differ significantly between the three groups. The mTOR gene is responsible for regulating cell growth and metabolism in response to growth factors and nutrients (Saxton and Sabatini 2017). In the current study, an increased immune response, which is an energy-demanding process that diverts nutrients from growth, and subsequently reduces performance, was observed (Emami et al., 2020). This might explain the non-significant differences in carcass traits between the treated groups and the control group.
Histomorphologic investigations of immune organs revealed that T2 and T3 displayed larger diameters of the white pulp of the spleen and follicle of the bursa compared to the control, with T3 recording the largest diameters for both organs. In addition, T2 demonstrated a significant boost of lymphocytic infiltration in the portal area of the liver, while T3 showed a mild increase in lymphocytic infiltration. From these observations, there was a general increase in the immunity induced by Bacillus probiotics, which come in agreement with the upregulation of TLR4 gene expression in the liver of treated groups. Mingmongkolchai and Panbangred (2018) reported that immune function modulations of host defense systems are necessary features of prospective probiotics. According to Duc et al. (2004) and Xu et al. (2012), Bacillus spores have been shown to improve innate immunity and macrophage phagocytosis.
Examinations of the small intestines of T2 birds revealed the appearance of lymph nodules in the lamina propria of the duodenum and jejunum, with a severe increase in the large intestine. Meanwhile, the same examinations in T3 birds revealed an increase in the lymphoid tissue of the lamina propria of the duodenum and a mild increase in the large intestine. Bacillus surface-associated proteins (such as S-layer proteins, aminopeptidase, flagellin, and cell envelop-bound metalloprotease) are specifically attached to mucin and fibronectin and may play a role in the probiotic strain’s adherence to the GIT (Sánchez et al., 2009). Bacterial adhesion to intestinal epithelial cells not only helps them colonize the gut but can also stimulate immune cells in the gut’s lymphoid tissue. According to Duc et al. (2004), Bacillus spores may penetrate microfold (M) cells and migrate through Peyer’s patches before being delivered to efferent lymph nodes. Antigen-presenting cells (dendritic cells and macrophages) are abundant in Peyer’s patches and are important in processing and presenting antigens to B cells for the synthesis of secretory IgA (sIgA), which are required for immunity against mucosal infections.
According to Sen et al. (2012), Bacillus generates micromorphological changes in the gut of broilers, increasing the villus height, as well as the villus-height-to-crypt-depth ratio, hence improving the small intestine’s nutritional digestibility and absorption capacity. This finding was in line with the significant difference we found in the length of the intestinal villi of the duodenum, jejunum, and ilium in T2 and T3 when compared to T1. Moreover, there was a significant difference in the crypt depth of the duodenum in T2 and T3 when compared to T1.
From the microbiological perspective, our results revealed the variable effects of Bacillus supplements on intestinal microbial counts during different stages of age. T2 showed the lowest counts of aerobic, anaerobic, and clostridial bacteria. There was no difference in intestinal microbial counts between T3 and the control (T1). These results indicated that there were significant reductions in gut bacterial counts, especially for Clostridia, in the multi-bacilli probiotic-treated group (T2). Several studies evaluated the effects of Bacillus on enhancing gut microflora and indicated that Bacillus produces peptides with powerful antimicrobial efficacy that suppress the multiplication of pathogenic bacteria like Clostridium perfringens and prevent the development of necrotic enteritis in broilers (Whelan et al., 2019; Sandvang et al., 2021). According to Sandvang et al. (2021), combining multiple strains of bacilli allowed the complementary interaction of the antibacterial effects of each single Bacillus strain. The results of the litter microbiological examination revealed that T2 and T3 displayed variable lower litter bacterial counts than the control (T1). These findings are in line with those of De Cesare et al. (2019), who reported that Bacillus spores showed the ability to inhabit the broilers’ litter and subsequently reduce the mean bacterial counts.
Physical examination of deep litter revealed that T2 showed the lowest level of litter moisture at the end of the experiment (day 35) but T3 showed high levels of litter moisture. High litter moisture activates bacterial growth and stimulates the anaerobic fermentation of litter (Payne et al., 2007; Sharma et al., 2017). These results are in line with the findings of Ribeiro et al. (2014) who reported that adding a high concentration of Bacillus to birds’ diets improved the moisture content of their excreta.
The nitrogen content of deep litter reported the lowest litter nitrogen levels in T2, followed by T3, which indicated adequate nitrogen digestion and absorption inside the intestinal tracts of birds. These results indicated the positive effect of supplementing broiler feed with Bacillus to enhance protein digestion and absorption and enhance the quality of the litter. Shojadoost et al. (2012) and Latorre et al. (2015) concluded that Bacillus feed supplements improved protein digestion and, subsequently, reduced the protein available to bacterial growth. Additionally, as litter nitrogen content drops, ammonia emissions in the broiler house are reduced and ammonia pollution of the environment is decreased. Ammonia, which is a significant air pollutant produced mainly from intensive animal and poultry production systems, possesses health hazards for animals and humans (Wang et al., 2009). Ferket et al. (2002) reported findings that were in line with these ones, as they indicated that ammonia emission is correlated to the intestinal microbiome and the utilization of nutrients. Jeong and Kim (2014) reported that broiler feed supplementation with Bacillus significantly reduced ammonia emissions from litter.
Conclusion
The long-term usage of antibiotic growth promoters (AGP) may induce bacterial resistance to antibiotics and raise antibiotic residual levels in animal products, which threatens both veterinary and public health. Probiotics are non-pathogenic microorganisms that are potentially important non-antibiotic alternatives. The present study concluded that the novel multi-strain Bacillus-based probiotic has a higher positive impact in promoting production profitability by encouraging bird growth and improving feed intake and FCR than a single-strain Bacillus-based probiotic. improving intestinal morphology and immune organs, upregulating immunity-related genes, all these inhibit harmful intestinal bacteria and sustain litter quality.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ahmat, M., Cheng, J., Abbas, Z., Cheng, Q., Fan, Z., Ahmad, B., Hou, M., Osman, G., Guo, H., Wang, J. & Zhang, R. 2021. Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics, 10, 1427. https://doi.org/10.3390/antibiotics10111427
Amoah, K., Dong, X.H., Tan, B.P., Zhang, S., Chi, S.Y., Yang, Q.H., Liu, H.Y., Yang, Y.Z. & Zhang, H. 2021. Effects of three probiotic strains (Bacillus coagulans, B. licheniformis and Paenibacillus polymyxa) on growth, immune response, gut morphology and microbiota, and resistance against Vibrio harveyi of northern whitings, Sillago sihama Forsskál (1775). Animal Feed Science and Technology, 277, 114958.
Aviagen. 2009. Arbor Acres: Broiler Nutrition Supplement. www.aviagen.com. Accessed 26 Dec 2021.
Bahrampour, K., Afsharmanesh, M. & Bami, M.K. 2020. Comparative effects of dietary Bacillus subtilis, Bacillus coagulans and Flavophospholipol supplements on growth performance, intestinal microflora and jejunal morphology of Japanese quail. Livestock Science, 239, 104089. https://doi.org/10.1016/j.livsci.2020.104089
Bancroft, J.D. & Gamble, M. 2008. Theory and practice of histological techniques. 6th Edition, Churchill Livingstone, Elsevier, China.
Bonos, E., Giannenas, I., Sidiropoulou, E., Stylianaki, I., Tzora, A., Skoufos, I., Barbe, F., Demey, V. & Christaki, E. 2021. Effect of Bacillus pumilus supplementation on performance, intestinal morphology, gut microflora, and meat quality of broilers fed different energy concentrations. Animal Feed Science and Technology, 274,114859.
Cutting, S.M. 2011. Bacillus probiotics. Food Microbiology, 28, 214-20.
Czarny, P., Wigner, P., Galecki, P. & Sliwinski, T. 2018. The interplay between inflammation, oxidative stress, DNA damage, DNA repair, and mitochondrial dysfunction in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 80, 309-21.
De Cesare, A., Caselli, E., Lucchi, A., Sala, C., Parisi, A., Manfreda, G. & Mazzacane, S. 2019. Impact of a probiotic-based cleaning product on the microbiological profile of broiler litters and chicken caeca microbiota. Poultry Science, 98, 3602-10.
Duc, L.H., Hong, H.A., Barbosa, T.M., Henriques, A.O. & Cutting, S.M. 2004.Characterization of Bacillus probiotics available for human use. Applied and Environmental Microbiology, 70, 2161-71.
Dumas, M.D., Polson, S.W., Ritter, D., Ravel, J., Gelb, Jr. J., Morgan, R. & Wommack, K.E. 2011. Impacts of poultry house environment on poultry litter bacterial community composition. PLoS One, 6, e24785.
Emami, N.K., Calik, A., White, M.B., Kimminau, E.A., & Dalloul, R.A. (2020). Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Frontiers in Veterinary Science, 7, 572142.
Esmaeilipour, O., Moravej, H., Shivazad, M., Rezaian, M., Aminzadeh, S. & Van Krimpen, M.M. 2012. Effects of diet acidification and xylanase supplementation on performance, nutrient digestibility, duodenal histology and gut microflora of broilers fed wheat-based diet. British Poultry Science, 53, 235-44.
Endres, J.R., Clewell, A., Jade, K.A., Farber, T., Hauswirth, J. & Schauss, A.G. 2009. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food and Chemical Toxicology, 47, 1231-8
ESVAC. 2017. Sales of veterinary antimicrobial agents in 30 European countries in 2015. Trends from 2010 to 2015. European Medicines Agency, Seventh European Surveillance of Veterinary Antimicrobial Consumption report (EMA/184855/2017).
Ferket, P.R., Parks, C.W., & Grimes, J.L. 2002. Mannan oligosaccharides versus antibiotics for turkeys. In Nutritional biotechnology in the feed and food industries: Proceedings of Alltech’s 18th Annual Symposium, Kentucky, USA, 43-64.
Gárate, I., Garcia-Bueno, B., Madrigal, J.L., Caso, J.R., Alou, L., Gomez-Lus, M.L., Micó, J.A. & Leza, J.C. 2013. Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biological Psychiatry, 73, 32-43.
Gernat, A.A., Santos, F.B. & Grimes, J.L. 2021. Alternative approaches to antimicrobial use in the Turkey industry: challenges and perspectives. German Journal of Veterinary Research, 1, 37–47. https://doi.org/10.51585/gjvr.2021.3.0018
Ghahri, H., Toloei, T. & Soleimani, B. 2013. Efficacy of antibiotic, probiotic, prebiotic and synbiotic on growth performance, organ weights, intestinal histomorphology and immune response in broiler chickens. Global Journal of Animal Scientific Research, 1, 25-41.
Ghoneim, N.H. & Hamza, D.A. 2017. Epidemiological studies on Clostridium perfringens food poisoning in retail foods. Revue Scientifique et Technique, 36, 1025-32.
Gu, S.B., Zhao, L.N., Wu, Y., Li, S.C., Sun, J.R., Huang, J.F. & Li, D.D. 2015. Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World Journal of Microbiology and Biotechnology, 31, 851-63. https://doi.org/10.1007/s11274-015-1838-x
Hafez, H.M. & Shehata, A.A. 2021.Turkey production and health: Current challenges. German Journal of Veterinary Research, 1, 3–14. https://doi.org/10.51585/gjvr.2021.0002
Hassan, N., Mostafa, I., Elhady, M.A., Ibrahim, M.A. & Amer, H. 2022. Effects of probiotic feed additives (biosol and Zemos) on growth and related genes in broiler chickens. Italian Journal of Animal Science, 21, 62-73
Hong, H.A., Duc, L.H. & Cutting, S.M. 2005. The use of bacterial spore formers as probiotics. FEMS Microbiology Reviews, 29, 813-35
Hung, A.T., Lin, S.Y., Yang, T.Y., Chou, C.K., Liu, H.C., Lu, J.J., Wang, B., Chen, S.Y. & Lien, T.F. 2012. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Animal Production Science, 52, 874-9. https://doi.org/10.1071/AN11332
Hussein, E.O., Ahmed, S.H., Abudabos, A.M., Aljumaah, M.R., Alkhlulaifi, M.M., Nassan, M.A., Suliman, G.M., Naiel, M.A. & Swelum, A.A. 2020. Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals, 10, 507.
Hussien, A., Ismael, E., Bawish, B. M., Kamel, S., Ismail, E. Y., El Bendari, E. K., & Fahmy, K. N. E. D. 2022. Response of Broiler Chickens to the Dietary Fortification of Bile Acid. Journal of Advanced Veterinary Research, 12(5), 582-587.
Jackson, M.L. 1973. Soil chemical analysis, Soil Chemical Analysis, Prentice Hall of India Pvt. Ltd., New Delhi, India, 498, 151-154.
Jeong, J.S. & Kim, I.H. 2014. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poultry Science, 93, 3097-103.
Kamel, S., Ibrahim, M., Awad, E.T., El-Hindi, H.M. & Abdel-Aziz, S.A. 2018. Differential expression of CYP2j2 gene and protein in Camelus dromedarius. Journal of Biological Regulators and Homeostatic Agents, (b), 32, 1473–7.
Kasas, A.H.E., Farag, I.M., Darwish, H.R., Soliman, Y.A., Nagar, E.M.E., Ibrahim, M.A., Kamel, S. & Warda, M. 2022. Molecular characterization of alpha subunit 1 of sodium pump (ATP1A1) gene in Camelus dromedarius: its differential tissue expression potentially interprets the role in osmoregulation. Molecular Biology Reports, 2, 1-3. https://doi.org/10.1007/s11033-022-07232-4
Kassambara, A. 2019. ggpubr R Package: ggplot2-Based Publication Ready Plots R package version 636. https://CRAN.R-project.org/package=ggpubr.
Khalaf, A.A., Hassanen, E.I., Ibrahim, M.A., Tohamy, A.F., Aboseada, M.A., Hassan, H.M. & Zaki, A.R. 2020. Rosmarinic acid attenuates chromium‐induced hepatic and renal oxidative damage and DNA damage in rats. Journal of Biochemical and Molecular Toxicology, 34, e22579.
Latorre, J.D., Hernandez-Velasco, X., Kuttappan, V.A., Wolfenden, R.E., Vicente, J.L., Wolfenden, A.D., Bielke, L.R., Prado-Rebolledo, O.F., Morales, E., Hargis, B.M. & Tellez, G. 2015. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Frontiers in Veterinary Science, 2, Article 25. https://doi.org/10.3389/fvets.2015.00025
LeBlanc, J.G., Chain, F., Martín, R., Bermúdez-Humarán, L.G., Courau, S. & Langella, P. 2017. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microbial Cell Factories, 16, 1–0. 10.1186%2Fs12934-017-0691-z
Liu, C., Radebe, S.M., Zhang, H., Jia, J., Xie, S., Shi, M.& Yu, Q. 2022. Effect of Bacillus coagulans on maintaining the integrity intestinal mucosal barrier in broilers. Veterinary Microbiology, 266, 109357.
Livak, K.J.& Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25, 402-8.
Lopes, M., Roll, V.F., Leite, F.L., Dai Prá, M.A., Xavier, E.G., Heres, T. & Valente, B.S. 2013. Quicklime treatment and stirring of different poultry litter substrates for reducing pathogenic bacteria counts. Poultry Science, 92, 638-44.
Maathuis, A., Keller, D.& Farmer, S. 2010. Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Beneficial Microbes, 1, 31-6. https://doi.org/10.3920/BM2009.0009
Manafi, M., Khalaji, S., Hedayati, M. & Pirany, N. 2017. Efficacy of Bacillus subtilis and bacitracin methylene disalicylate on growth performance, digestibility, blood metabolites, immunity, and intestinal microbiota after intramuscular inoculation with Escherichia coli in broilers. Poultry Science, 96, 1174-83.
Marcu, A., Vacaru-Opriş, I., Dumitrescu, G., Ciochină, L.P., Marcu, A., Nicula, M., Peţ, I., Dronca, D., Kelciov, B.& Mariş, C. 2013. The influence of genetics on economic efficiency of broiler chickens growth. Animal Science and Biotechnologies, 46, 339-46.
Mazkour, S., Shekarforoush, S.S., Basiri, S., Nazifi, S., Yektaseresht, A. & Honarmand, M. 2020. Effects of two probiotic spores of Bacillus species on hematological, biochemical, and inflammatory parameters in Salmonella Typhimurium infected rats. Scientific Reports, 10, 1-1.
Mehdi, Y., Létourneau-Montminy, M.P., Gaucher, M.L., Chorfi, Y., Suresh, G., Rouissi, T., Brar, S.K., Côté, C., Ramirez, A.A. & Godbout, S. 2018. Use of antibiotics in broiler production: Global impacts and alternatives. Animal Nutrition, 4, 170-8. https://doi.org/10.1016/j.aninu.2018.03.002
Mingmongkolchai, S. & Panbangred, W. 2018. Bacillus probiotics: an alternative to antibiotics for livestock production. Journal of Applied Microbiology, 124, 1334-46.
Moeller, R., Setlow, P., Reitz, G. & Nicholson, W.L. 2009. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Applied and Environmental Microbiology, 75,5202-8.
OIE. 2022. Newcastle disease (infection with Newcastle disease virus). Chapter 3.3.14. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Version adopted in May 2021).
Payne, J.B., Osborne, J.A., Jenkins, P.K. & Sheldon, B.W. 2007. Modeling the growth and death kinetics of Salmonella in poultry litter as a function of pH and water activity. Poultry Science, 86, 191-201.
Qin, J., Zhao, B., Wang, X., Wang, L., Yu, B., Ma, Y., Ma, C., Tang, H., Sun, J. & Xu, P. 2009. Non-sterilized fermentative production of polymer-grade L-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2–6. PloS One, 4, e4359.
Rashidi, N., Khatibjoo, A., Taherpour, K., Akbari-Gharaei, M. & Shirzadi, H. 2020. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B1. Poultry Science, 99, 5896-906.
Reis, S. A., Conceição, L. L., Rosa, D. D., Siqueira, N. P. & Peluzio, M. C. G. 2017. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutrition Research Reviews, 30, 36-49. https://doi.org/10.1017/S0954422416000226
Ribeiro, Jr. V., Albino, L.F., Rostagno, H.S., Barreto, S.L., Hannas, M.I., Harrington, D., De Araujo, F.A., Ferreira, Jr. H.C. & Ferreira, M.A. 2014. Effects of the dietary supplementation of Bacillus subtilis levels on performance, egg quality and excreta moisture of layers. Animal Feed Science and Technology, 195, 142-6.
Rozs, M., Manczinger, L., Vágvölgyi, C. & Kevei, F. 2001. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiology Letters, 205, 221-4.
Sandvang, D., Skjoet-Rasmussen, L., Cantor, M.D., Mathis, G.F., Lumpkins, B.S. & Blanch, A. 2021. Effects of feed supplementation with 3 different probiotic Bacillus strains and their combination on the performance of broiler chickens challenged with Clostridium perfringens. Poultry Science, 100, 100982.
Sánchez, B., Arias, S., Chaignepain, S., Denayrolles, M., Schmitter, J.M., Bressollier, P. & Urdaci, M.C. 2009. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology, 1; 155, 1708-16.
Saxton, R.A. & Sabatini, D.M. 2017. mTOR signaling in growth, metabolism, and disease. Cell, 168, 960-76.
Sen, S., Ingale, S.L., Kim, Y.W., Kim, J.S., Kim, K.H., Lohakare, J.D., Kim, E.K., Kim, H.S., Ryu, M.H., Kwon, I.K. & Chae, B.J. 2012. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Research in Veterinary Science, 93, 264-8.
Sharma, N.K., Choct, M., Dunlop, M.W., Wu, S.B., Castada, H.Z. & Swick, R.A. 2017. Characterization and quantification of changes in odorants from litter headspace of meat chickens fed diets varying in protein levels and additives. Poultry Science, 96, 851-60.
Sharma, U., Pal, D. & Prasad, R. 2014. Alkaline phosphatase: An overview. Indian Journal of Clinical Biochemistry, 29, 269-78
Shojadoost, B., Vince, A.R. & Prescott, J.F. 2012. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Veterinary Research, 43, 1-2.
Team, R.C. 2019. R: A language and environment for statistical computing. Vienna, Austria.
Tellez-Isaias, G., Christine, N.V., Brittany, D.G., Callie, M.S., Lucas, E.G., Roberto, S., Thaina, L.B., Lesleigh, C.B., Makenly, E.C., Aaron, J.F., Ruff, J., Hernandez-Velasco, X. & Billy, M. H. 2021. Developing probiotics, prebiotics, and organic acids to control Salmonella spp. in commercial turkeys at the University of Arkansas, USA. German Journal of Veterinary Research, 3, 7–12. https://doi.org/10.51585/gjvr.2021.3.0014
Upadhaya, S.D., Rudeaux, F. & Kim, I.H. 2019. Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poultry Science, 98, 4722-8. https://doi.org/10.3382/ps/pez184
Upadhaya, S.D., Rudeaux, F. & Kim, I.H. 2019. Effects of inclusion of Bacillus subtilis (Gallipro) to energy-and protein-reduced diet on growth performance, nutrient digestibility, and meat quality and gas emission in broilers. Poultry Science, 98, 2169-78.
Wang, Y., Cho, J.H., Chen, Y.J., Yoo, J.S., Huang, Y., Kim, H.J. & Kim, I.H. 2009. The effect of probiotic BioPlus 2B® on growth performance, dry matter and nitrogen digestibility and slurry noxious gas emission in growing pigs. Livestock Science, 120, 35-42.
Wealleans, A.L., Sirukhi, M. & Egorov, I.A. 2017. Performance, gut morphology and microbiology effects of a Bacillus probiotic, avilamycin and their combination in mixed grain broiler diets. British Poultry Science, 58, 523-9.
Whelan, R.A., Doranalli, K., Rinttilä, T., Vienola, K., Jurgens, G. & Apajalahti, J. 2019. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poultry Science, 98, 3450-63.
Wickham, H. 2016. ggplot2: elegant graphics for data analysis. Springer. https://doi.org/10.1007/978-3-319-24277-4
Xing, S.C., Chen, J.Y., Cai, Y.F., Huang, C.B., Liao, X.D. & Mi, J.D. 2021. Bacillus coagulans R11 consumption influenced the abundances of cecum antibiotic resistance genes in lead-exposed laying hens. Environmental Pollution, 274, 116562.
Xu, X., Huang, Q., Mao, Y., Cui, Z., Li, Y., Huang, Y., Rajput, I.R., Yu, D. & Li, W. 2012. Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiology and Immunology, 56, 817-24.
Yun, W., Lee, D.H., Choi, Y.I., Kim, I.H. & Cho, J.H. 2017. Effects of supplementation of probiotics and prebiotics on growth performance, nutrient digestibility, organ weight, fecal microbiota, blood profile, and excreta noxious gas emissions in broilers. Journal of Applied Poultry Research, 26, 584-92. https://doi.org/10.3382/japr/pfx033
Zhang, S., Zhong, G., Shao, D., Wang, Q., Hu, Y., Wu, T., Ji, C. & Shi, S. 2021. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poultry Science, 1, 100, 100935. https://doi.org/10.1016/j.psj.2020.12.032
Zhou, M., Zeng, D., Ni, X., Tu, T., Yin, Z., Pan, K. & Jing, B. 2016. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids in Health and Disease, 15, 1-0.
Zhu, C., Gong, L., Huang, K., Li, F., Tong, D. & Zhang, H. 2020. Effect of heat-inactivated compound probiotics on growth performance, plasma biochemical indices, and cecal microbiome in yellow-feathered broilers. Frontiers in Microbiology, 11, Article 585623. https://doi.org/10.3389/fmicb.2020.585623
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ebtihal M. M. Elleithy contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, as well as writing the manuscript. All authors read and approved the final manuscript.
Basma M. Bawish contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, the writing of the manuscript, revision, as well as final editing All authors read and approved the final manuscript.
Shaimaa Kamel contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, as well as writing the manuscript. All authors read and approved the final manuscript.
Elshaimaa Ismael contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, the writing of the manuscript, revision, as well as final editing. All authors read and approved the final manuscript.
Dina W. Bashir contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, as well as writing the manuscript. All authors read and approved the final manuscript.
Dalia Hamza contributed to the collection of samples, the design of the experiment, the analysis, and interpretation of the data, the writing the manuscript, revision, as well as final editing. All authors read and approved the final manuscript.
Khaled Nasr El-din Fahmy contributed to the collection of samples, the design of the experiment, the analysis, the interpretation of the data, and the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee guidelines, Faculty of Veterinary Medicine, Cairo University, Egypt. (Ethical reference No: Vet CU28/04/2021/280). All procedures and methods that have been used in this study were performed in compliance with the guidelines and regulations of the research ethics committee in the Faculty of Veterinary Medicine, Cairo University. Al methods are reported in accordance with ARRIVE.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elleithy, E.M.M., Bawish, B.M., Kamel, S. et al. Influence of dietary Bacillus coagulans and/or Bacillus licheniformis-based probiotics on performance, gut health, gene expression, and litter quality of broiler chickens. Trop Anim Health Prod 55, 38 (2023). https://doi.org/10.1007/s11250-023-03453-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03453-2