Abstract
Foot-and-mouth disease (FMD) is a highly contagious disease of cloven-hoofed animals. In Uganda, FMD outbreaks are mainly controlled by ring vaccination and restriction of animal movements. Vaccination stimulates immunity and prevents animals from developing clinical signs which include lameness, inappetence, and decreased production. Ring vaccination and restriction of animal movements have, however, not successfully controlled FMD in Uganda and outbreaks reoccur annually. The objective of this study was to review the use of FMD virus (FMDV) vaccines and assess the effectiveness of vaccination programs for controlling FMD in Uganda (2001–2010), using retrospective data. FMD vaccine distribution patterns in Uganda (2001–2010) matched occurrence of outbreaks with districts reporting the highest number of outbreaks also receiving the largest quantity of vaccines. This was possibly due to “fire brigade” response of vaccinating animals after outbreaks have been reported. On average, only 10.3 % of cattle within districts that reported outbreaks during the study period were vaccinated. The average minimum time between onset of outbreaks and vaccination was 7.5 weeks, while the annual cost of FMDV vaccines used ranged from US $58,000 to 1,088,820. Between 2001 and 2010, serotyping of FMD virus was done in only 9/121 FMD outbreaks, and there is no evidence that vaccine matching or vaccine potency tests have been done in Uganda. The probability of FMDV vaccine and outbreak mismatch, the delayed response to outbreaks through vaccination, and the high costs associated with importation of FMDV vaccines could be reduced if virus serotyping and subtyping as well as vaccine matching were regularly done, and the results were considered for vaccine manufacture.
Similar content being viewed by others
Introduction
Foot-and-mouth disease (FMD) is a highly contagious viral disease of wild and domestic cloven-hoofed animals (Alexandersen 2005, pp. 9–12). The disease in livestock is associated with serious socioeconomic consequences due to marked loss in production and/or death of affected animals and interference with marketing of livestock products (Perry 2007, pp. 238–241; Arzt et al. 2011, pp. 291–304). These consequences of FMD are more prominent in developing countries in sub-Saharan Africa, and disease-free status is an indication of development since most developed countries are FMD free (Pattnaik et al. 2012, pp. 132–147). FMD is caused by a virus of the genus Aphthovirus and family Picornaviridae (Arzt et al. 2011, pp. 291–304). Seven serotypes of the FMD virus (FMDV) have been identified, namely, O, A, C, Asia 1, and Southern African Territories (SAT) 1, SAT 2, and SAT 3 (Belsham et al. 2011). Vaccination against one FMDV serotype does not usually protect animals against other serotypes of the virus or other strains of the same serotype (Pattnaik et al. 2012, pp. 132–147).
The first FMD outbreak in Uganda was recorded in 1953, and since then, many outbreaks involving all FMDV serotypes, except for Asia 1, have been reported in the country (Ayebazibwe et al. 2010b). The disease is reported annually in Uganda’s 11.4 million cattle with previous studies showing incursions of serotypes O, A, SAT 1, and SAT 2 (Mwiine et al. 2010a, pp. 89–96). In Uganda, FMD outbreaks are mainly controlled by ring vaccination and quarantine that involves restriction of animal movements from affected districts (Mwiine et al. 2010b, pp. 365–374). The strategy of ring vaccination involves immunizing animals in areas surrounding a known source of infection (Keeling et al. 2003, pp. 136–141). In a number of countries, but not in Uganda, ring vaccination is usually implemented alongside the slaughter of sick and in-contact animals to prevent spread of the disease (Pattnaik et al. 2012, pp.132–147). If used strategically, vaccination can create a barrier between infected and disease-free areas, provided that FMDV vaccine serotypes and subtypes match with those causing outbreaks in a given area (Doel 2003, pp. 81–99). However, control strategies, based on vaccination and quarantine, have not stopped the occurrence of FMD outbreaks in Uganda, which underscores the need for evaluation of vaccines and vaccination programs in place. Previous reports have described the spatial distribution of FMD outbreaks in Uganda (Ayebazibwe et al. 2010a, pp. 1547–1559). However, there are no studies that have assessed vaccination programs and vaccines used to control FMD outbreaks in the country in the recent past. The objective of this study was to review the use of FMDV vaccines and assess the effectiveness of vaccination programs for controlling FMD in Ugandan livestock over a period of 10 years using retrospective data. The specific objectives of the study were to describe FMD outbreaks occurrence and vaccine usage in Uganda’s livestock, to evaluate the effectiveness of the vaccination programs used to control FMD outbreaks in the country, and to compare the field and vaccine FMDV serotypes in the different regions of the country.
Materials and methods
Data source and type
Retrospective data on FMD outbreaks in Ugandan livestock were obtained from the National Animal Disease Diagnostics and Epidemiology Centre, Ministry of Agriculture Animal Industry and Fisheries (MAAIF), Entebbe, Uganda. The data used were for a 10-year period (2001–2010) with the expectation that inferences based on these data would provide an insight into the current patterns of FMD occurrence in the country, given that outbreaks occur every year. Also, FMD is a notifiable disease in Uganda, and as such, all outbreaks of the disease must be reported to MAAIF immediately. The information obtained this way therefore reflects the disease situation in the country. The variables of interest included: “time” (year, month, and day) and “place” (subcounty, district, and region) where the outbreaks occurred, the districts reporting FMD outbreaks, livestock populations (obtained from the 2008 National Livestock Census and carried out jointly by the Uganda Bureau of Statistics (UBOS) and MAAIF (UBOS/MAAIF 2009, pp. 41), vaccine cost, vaccine quantities, and vaccine serotypes of imported FMD vaccines as well as vaccine distribution records.
All vaccines bought and distributed by MAAIF are entered into a stock ledger, and these data provided information about FMD vaccine distribution in Uganda. The price list of Kenya Veterinary Vaccines Production Institute was used to obtain the cost of the imported FMD vaccines. Additionally, data from the serotyping of Ugandan FMD virus field isolates tested at the Pirbright World Reference Laboratory, Surrey, UK, were obtained and compared to serotypes in the vaccines imported. Additional variables created included percentage of cattle vaccinated, minimum time in weeks between outbreaks and vaccination onset (categories 1 = 1 week, 2 = 2–4 weeks, 3 = 5–12 weeks, and 4 = >12 weeks), and vaccine type (type 1 = bivalent vaccines, type 2 = trivalent/quadrivalent vaccines). The proportion of livestock vaccinated was calculated by dividing quantity of FMDV doses distributed to districts after reported FMD outbreaks by district livestock populations. The minimum time of onset of vaccination was deduced from the dates when outbreaks were reported to the dates of vaccine distribution. The phrase “minimum time of onset of vaccination” was used because delivery of vaccines and onset of vaccination in the districts is usually delayed by transport costs and inadequate staff. The response variable was outbreak sum which was computed as the number of additional outbreaks observed within each district. For descriptive purposes, the study area was divided into seven regions, namely, Northern, Western, Eastern, Central, Southwestern, Northeastern, and Northwestern (Ayebazibwe et al. 2010a, pp. 1547–1559).
Data analysis
The data obtained were entered into Microsoft Excel 2010 spreadsheet, and descriptive statistics were generated. Additionally, a negative binomial regression was performed using the GENMOD procedure of SAS using outbreak sum as the outcome variable with all the other variables acting as independent variables. Data on FMD outbreak occurrence, FMD vaccine distribution, and cattle populations in the various districts of Uganda were imported into Arc-GIS software spatial, and maps of FMD outbreak occurrence in the country were generated. The proportion of cattle vaccinated, minimum time between outbreaks and vaccination onset, as well as the cost of FMDV vaccines were used to assess the effectiveness of vaccination programs in Uganda. Vaccine effectiveness was equated to FMD outbreak sum computed as the number of additional outbreaks observed within each district.
Results
Distribution of foot-and-mouth disease outbreaks in Uganda, 2001–2010
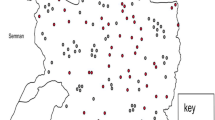
The highest number of FMD outbreaks occurred in the Central Region (n = 121; 34 %), followed by Eastern (n = 121; 19 %), Southwestern (n = 121; 17 %), Northern (n = 121; 13 %), Western (n = 121; 13 %), Northeastern (n = 121: 3 %), and Northwestern (n = 121; 1 %). A total of 121 outbreaks were reported during the study period. The region statistically significantly influenced the occurrence of FMD outbreaks (P < 0.0001) in the country with the majority of the outbreaks occurring in the central region comprising of Nakasongola/Luwero and Rakai districts (Fig. 1).
FMD vaccine distribution in Uganda, 2001–2010
The Central Region received the highest percentage (43 %) of FMDV vaccine doses, followed by Southwestern (35 %), Western (9 %), Eastern (7 %), Northern (3 %), Private Farms (2 %), Northeastern (1 %), and lastly, Northwestern (0.3 %). A total of 5,690,209 FMD vaccine doses were distributed throughout the study period. Generally, regions, where high numbers of outbreaks occurred, received the greatest quantities of FMD vaccine; for instance, majority of outbreaks (36 %), as well as the greatest percentage of vaccine doses used (43 %), occurred in the central region. However, a different trend was observed in the eastern and northern regions of Uganda, where low percentages of FMD vaccine doses were distributed [7 % (eastern) and 3 % (northern)], despite high percentages of FMD outbreaks [18 % (eastern region) and 13 % (northern)].
Comparison between cattle population, FMD outbreaks occurrence, and FMD vaccine distribution, 2001–2010
The spatial pattern of FMD vaccine distribution during the study period (2001–2010) was similar to that of FMD outbreaks and the cattle population. As shown in Fig. 2a, b, and c, the southwestern (Mbarara) and central regions (Nakasongola and Kiboga), which had the highest cattle population, also reported the highest number of outbreaks and received the largest quantities of FMD vaccines during the study period. The exceptions were the northeastern region that had high cattle numbers but reported few outbreaks and likewise received low amounts of FMD vaccines, and the northern region which had a low cattle population but reported high numbers of FMD outbreaks and paradoxically received low quantities of FMD vaccine doses.
a A map of Uganda showing cattle population in the different regions (Central, Western, Eastern, Northern, Northeastern, Northwestern, and Southwestern). The increase in intensity of the gray scale shade corresponds with the cattle population size in districts within the regions. Areas with 0–65,000 cattle represented with the least intense gray shade; areas with 65,001–150,000, 150,001–250,000, and 250,001–350,000 cattle are shown with increasing intensity of gray with increase in cattle population, and areas with over 350,000 cattle are shown with a black shade. b A map of Uganda showing the number of foot-and-mouth that occurred in the different regions (Central, Western, Eastern, Northern, Northeastern, Northwestern, and Southwestern) from 2001 to 2010. Areas that reported no outbreaks are in white, and those that had outbreaks have gray scale shades. The intensity of the gray scale shade increases with number of FMD outbreaks from 1, 2, 3–6, to 7–14. c A map of Uganda showing the quantity of foot-and-mouth disease virus vaccine distributed to the different districts in the Central, Western, Eastern, Northern, Northeastern, Northwestern, and Southwestern regions of Uganda from 2001 to 2010. The size of the bubble directly proportional to the quantity of FMDV vaccines distributed, and ranges from 0 through 1–5,000; 5,001–10,000; 10,001–20,000; 20,001–50,000; 50,001–100,000; 100,001–200,000; to 200,001–1,500,000 are represented by the biggest bubble
Effectiveness of vaccination programs for the control of foot-and-mouth disease outbreaks in Uganda (2001–2010)
All FMD vaccines distributed during the 10-year-study period were used on cattle, and there were no records of vaccine usage in other animals like sheep, goats, and pigs. The proportion of cattle vaccinated per year in districts that reported outbreaks are shown in Fig. 3 and ranged from 2.1 to 21.2 % (10.3 % average). These proportions were not statistically significantly different by district given the FMD outbreaks (P = 0.869). The minimum time from onset of FMD outbreaks to intervention or response through vaccination ranged from 1 to 40 weeks with an average of 7.5 weeks (Table 1). The minimum time taken to respond to outbreaks through vaccination statistically significantly influenced occurrence of FMD outbreaks (P < 0.0001) (Tables 2 and 3).
Comparison of the percentage of cattle vaccinated against foot-and-mouth disease (FMD) with the number of FMD outbreaks that occurred per year from 2001 to 2010. The black bars show the number of foot-and-mouth disease outbreaks reported per year, while the gray bars show the percentage of cattle vaccinated in districts that reported outbreaks per year
The cost of FMD vaccines imported during the study period ranged from US $58,000 in 2003 to US $1,088,820 in 2009 (Table 2). Generally, the quantities of FMD vaccines imported increased with the increase in the number of FMD outbreaks except for the years 2003, 2005, 2007, and 2009. However, 15 outbreaks occurred in 2003 when the lowest quantities of FMD vaccine were imported, while 11 outbreaks occurred in 2009 when the highest quantities of vaccines were used (Table 2).
Comparison of FMD field outbreak and FMD vaccine serotypes in Uganda, 2001–2010
Serotyping was done for only nine out of the 121 (7.4 %) FMD outbreaks serotypes which occurred in Uganda during the study period. Serotypes of FMD virus identified during the study period are shown in Table 1. From 2001 to 2010, 93 % of the vaccines doses were of trivalent composition and included serotypes O/SAT1/SAT2; 0.5 % contained serotypes A/O/SAT2; 0.7 % were quadrivalent (O/A/SAT1/SAT2), and 5.7 % were bivalent (O/SAT1). There was no statistical difference between the vaccine types (bivalent, trivalent, and quadrivalent vaccines) used (P = 0.8659) in regard to preventing FMD outbreaks.
Negative binomial regression
Results of the GENMOD procedure showed that the region and the minimum time between occurrence of outbreaks and vaccination onset were highly significant for the occurrence of additional FMD outbreaks as shown in Table 3.
Discussion
The total number of FMD outbreaks (121) in this study is different from that reported by Ayebazibwe et al. 2010a (131) who used subcounties as the reporting unit. This study considered that outbreaks occurring concurrently in subcounties of a given district are the same because vaccination and restriction of animal movement are implemented at district level, and thus, FMD spread within a given district from one subcounty is highly probable.
The highest number of FMD outbreaks occurred in the central region, followed by the eastern and southwestern regions. This concurs with Ayebazibwe et al. (2010a) who reported that the central, eastern, and western regions had the highest number of FMD outbreaks. Additionally, the highest quantity of FMDV vaccine doses was distributed to the central region. Within the central region, many of the outbreaks occurred in Rakai, Luwero/Nakasongola, and Mubende (Fig. 1). This is because Rakai is located along the Uganda–Tanzania boarder where there is frequent and uncontrolled movement of cattle along the border which predisposes cattle to diseases including FMD (Ayebazibwe et al. 2010a, pp. 1547–1559).
Additionally, several subcounties of Luwero, Nakagongola, and Mubende suffer long periods of drought, which are usually accompanied with animal movements and loss of condition that predisposes animals to FMD (Ayebazibwe et al. 2010a, pp. 1547–1559). Furthermore, Mubende, Luwero, and Nakasongola are popular for livestock trade, especially towards the dry season when farmers anticipate cattle losses due to scarcity of pastures and water. Livestock from different subcounties and neighboring districts are brought to a market place which eases disease spread and may possibly be an additional explanation to the high number of FMD outbreaks in the districts. Similarly, the eastern region has a communal grazing system coupled to its proximity to the Kenyan boarder where animal movements across the border are rampant. It is likely that introduction of animals with FMD may be responsible for the high number of FMD outbreaks (19 %) (Ayebazibwe et al. 2010a, pp. 1547–1559).
However, eastern region received less FMDV vaccine doses (7 %) than other regions where fewer outbreaks occurred. For example, southwestern region received 35 % of the vaccine doses distributed, although it reported fewer outbreaks (17 %). This is because the communal grazing in the eastern region may have frustrated government control strategies (Turner 2005). In communal grazing, cattle are moved to a grazing land that has pastures throughout the year, especially during the dry season. This practice may hinder disease control efforts like vaccination because it makes it hard to trace animal owners who, most of the times, leave the animals to herdsmen. Vaccination of livestock requires the owner’s consent, and since most FMD outbreaks occur in the dry season when communal grazing is practiced, fewer animals were vaccinated. Additionally, herd sizes are larger in the western region than in the eastern region. Thus, the farmer-perceived-risk is bigger in western than in eastern region.
The southwestern region had lower numbers of FMD outbreaks than the eastern and central regions, probably due to the paddock grazing system practiced by the majority of farmers. The high quantities of FMDV vaccines (35 %) distributed to the southwestern region may indicate preoutbreak FMD vaccination probably done when outbreaks had been reported in other regions. The northwestern region had the lowest number of FMD outbreaks possibly due to the low cattle population and the natural barrier provided by the River Nile which limits cattle movement in to the region, thus reducing chances of FMD transmission.
The spatial pattern of FMDV vaccine distribution during the study period was similar to that of outbreaks and cattle population (Fig. 2a–c), probably due to the governments’ fire brigade response of vaccinating cattle against FMD after outbreaks are reported. The trend was, however, different in the northern and northeastern regions. The high number of FMD outbreaks with limited vaccination in the northern region may have been due to the Lord’s Resistance Army rebellion that caused insecurity (Turner 2005) in the area, thus hindering vaccination exercises.
The few outbreaks reported in northeastern Uganda, despite its high cattle population, may possibly be due to underreporting of FMD outbreaks. The northeastern region, also known as Karamoja region, has inadequate veterinary services, and the transhumance way of life of the Karamojong has contributed to their reliance on traditional ethnoveterinary knowledge to treat and prevent livestock diseases (Grade et al. 2009, pp. 273–293). Thus, it is likely that most outbreaks were not reported to the veterinarian at the districts as most pastoralists may have resorted to their local ethnoveterinary knowledge to treat symptoms of FMD. All the above explain that the region was highly significant for the occurrence of additional FMD outbreaks within the study period.
The proportions of cattle vaccinated (2.1 to 21.2 %) after outbreaks were not statistically significant for the occurrence of FMD outbreaks. Vaccination programs are effective when a large proportion of the population is protected, usually at least 72 % of the susceptible cattle need to be vaccinated (Keeling et al. 2003, pp. 136–141). Therefore, vaccination of cattle below the recommended levels may not have any impact on the occurrence of the disease since no herd immunity is developed. Limited ring vaccination could also be responsible for outbreaks occurring within 3 months after previous outbreak because unvaccinated animals may be sources of subsequent infections (Keeling 2003, pp. 136–141). Herd immunity results when protective antibody levels have developed in 80–85 % of the susceptible animals (Ayebazibwe et al. 2010b). The low proportion of cattle vaccinated after FMD outbreaks thus reduces the effectiveness of postoutbreak FMD vaccination.
No records were available about FMDV vaccine distributed for pigs and small ruminants. This agrees with Balinda et al. (2009) who noticed that control of foot-and-mouth disease involves vaccination of cattle in affected area, leaving out small ruminants and pigs. Small ruminants may be responsible for the maintenance of FMDV on farms since cattle, sheep, and pigs are, most of the times, reared together (León 2011, pp. 36–49). This may play a major role in the epidemiology of foot-and-mouth disease. FMD vaccination programs should therefore include pigs and small ruminants.
The minimum time between FMD outbreaks and onset of vaccination ranged from 1 to 40 weeks. The average minimum time for onset of vaccination after reported FMD outbreaks was 7.5 weeks which, according to Chowell et al. (2006), is too high for effective postoutbreak vaccination. Chowell et al. (2006) noticed that the time required for effective postoutbreak vaccination is brief, starting at day 5 for high potency vaccines and day 12 for regular vaccines. He further reported that only marginal reductions in FMD cases can be achieved if vaccination is done after the 15th day into the outbreak (Chowell et al. 2006, pp. 73–87). Thus, time taken to respond to outbreaks through vaccination is highly significant for the effectiveness of FMD control. Most animals should therefore be vaccinated before the 15th day of the outbreak.
The cost of FMDV vaccines used (US $58,000–1,088,820) is high. This may hinder FMD control efforts, given the small sized budgets that Uganda and other developing countries operate with. Importation of FMDV vaccines delays the onset of vaccination activities. This is evidenced by the general delay in onset of vaccination in districts that reported outbreaks from 2001 to 2010 as shown in Table 1. Because of the occurrence of multiple outbreaks every year as described in Table 1, it is possible that FMDV vaccines run out of stock and must be ordered from other countries, resulting in delayed response as well as FMD spread to unprotected herds. Outbreaks should therefore be responded to in the shortest time possible to reduce the risk of spread of FMD (Windsor et al. 2011, pp. 421–433). Furthermore, the possibility of inadequate temperature conditions during delivery, storage, and transportation of vaccines to the field cannot be ruled out. The above possible irregularities underscore the importance of local FMDV vaccine development and manufacture, particularly with the recent increase in the number of public–private partnerships and intergovernmental organizations investing in biotechnology, especially animal disease diagnostics and vaccine production (Ecuru and Naluyima 2010, pp. 133–139). A good example is the newly established Uganda Industrial Research Institute, a public–private partnership making Newcastle vaccines (UIRI 2011).
Serotyping was done for only nine of the 121 outbreaks that occurred during the study period. This was possibly due to lack of infrastructure and personnel to identify the serotype of FMDV in samples (Belsham et al. 2011). Because Uganda lacks such infrastructure, the samples were transported to reference laboratories, i.e., the World Reference Laboratory, Pirbright, UK (Belsham et al. 2011). This involves significant costs of transportation, and the samples have to be collected and stored under appropriate conditions until they are submitted to the reference laboratories (Belsham et al. 2011). This is difficult due to the high ambient temperatures and the distances from the points of collection to the national laboratory (Belsham et al. 2011). It is, therefore, crucial that laboratory facilities and personnel be established to carry out regular serotyping of FMD viruses causing outbreaks (León 2011, pp. 36–49).
Multivalent FMDV vaccines were used to control outbreaks during the study period with 93 % of which contained O/SAT1/SAT2. This was done to cover all the possible serotypes that are likely to occur in field situations, given the limitations of FMDV serotyping in Uganda. Since all vaccines were multivalent and contained serotypes identified in 9/121 outbreaks, the type of vaccine used had no statistically significant influence on occurrence of additional FMD outbreaks. However, outbreaks occurred annually despite the rigorous vaccination efforts. This may be due to a mismatch in field and vaccine FMDV subtypes and variants, given the wide genetic variation of the virus (León 2011, pp. 36–49). This wide genetic heterogeneity presents the greatest obstacle to control efforts by vaccination (León 2011, pp. 36–49). Additionally, vaccine potency tests should be done to determine the effectiveness of vaccines used. Uganda’s endemic status of FMD affects its gross domestic product and leads to loss of revenue from the sale of animals and animal products to FMD-free countries (Perry 2007, pp. 238–241). It is, therefore, important that regular virus serotyping and subtyping, vaccine matching, as well as monitoring of antibodies in vaccinated populations be undertaken for creation of FMD-free zones as well as a basis for vaccine manufacture (Pattnaik et al. 2012, pp. 132–147).
The long time for onset of vaccination after FMD outbreaks (7.5 weeks), the low proportion vaccinated cattle (10.3 %) in affected districts, and unsettled grazing systems may contribute to the endemicity of FMD in Uganda. Although multivalent FMD vaccines were used, the vaccine and field virus subtypes and variants may not be homologous, underscoring the need for regular virus serotyping and subtyping, vaccine matching, as well as monitoring of antibody production in vaccinated cattle as a basis for creation of disease-free zones as well as vaccine production.
References
Alexandersen, S., Mowat, N., 2005. Foot-and-mouth disease: host range and pathogenesis, Current Topics in Microbiology and Immunology, 288, 9–42
Arztl, J., Juleff, N., Zhang, Z. and Rodriguez, L. L., 2011. The pathogenesis of Foot-and-mouth disease I: viral pathways in cattle, Transboundary and Emerging Diseases. 58, 291–304
Ayebazibwe, C., Mwiine, F. N., Tjørnehøj K., Balinda, S. N., Muwanika, V. B., Ademun, O. R., Belsham, G. J., Normann, J., Siegismund, H. R., Alexandersen, S., 2010 b. The role of African buffalos (Syncerus caffer) in the maintenance of foot-and-mouth disease in Uganda, BioMed Central Veterinary Research, 6, 54, doi:10.1186/1746-6148-6-54
Ayebazibwe, C., Tjørnehøj, K., Mwiine, F. N., Muwanika, V. B., Ademun, O. A. R., Siegismund, H. R., Alexandersen, S., 2010a. Patterns, risk factors and characteristics of reported and perceived foot-and-mouth disease (FMD) in Uganda, Tropical Animal Health and Production, 42(7), 1547–1559
Balinda, S. N., Tjørnehøj, K., Muwanika, V. B., Sangula1, A. K., Mwiine, F. N., Ayebazibwe, C., Masembe, C., Siegismund, H. R. and Alexandersen, S. (2009). Prevalence Estimates of Antibodies Towards Foot-and-Mouth Disease Virus in Small Ruminants in Uganda, Transboundary and Emerging Diseases, 56, 362–371
Belsham, J. G., Jamal, S. M., Tjørnehøj, K., Bøtner, A., 2011. Rescue of Foot-and-Mouth Disease Viruses that are Pathogenic for Cattle from Preserved Viral RNA Samples. PLoS ONE, 6(1), e14621. doi:10.1371/journal.pone.0014621
Chowell, G., Rivas, A. L., Hengartner, N. W., Hyman, J. M., and Castillo-Chavez, C., 2006. Critical response to post-outbreak vaccination against foot-and-mouth disease. In: B.A. Gomel, C. Castillo-Chavez, R.E. Mickens, P.D. Clemence (eds), Mathematical studies on human disease dynamics: Emerging paradigms and challenges, Utah, 2006, (American Mathematical Society; Contemporary Mathematics 410), 73–87
Doel, T. R., 2003. FMD Vaccines, Virus Research, 91, 81–99
Ecuru, J. and Naluyima, H., 2010. Biotechnology developments in Uganda and associated challenges, African Crop Science Journal, 18 (4), 133–139
Grade, T., Tabuti, R. S. J., Van Damme, P., 2009. Ethnoveterinary knowledge in pastoral Karamoja, Kampala Uganda, Ethnopharmacology, 122, 273–293
Keeling, M. J., Woolhouse, M. E. J., May, R. M., Davies G., Grenfellk, B. T., 2003. Modeling vaccination strategies against foot-and-mouth disease, Nature, 421, 136–141
León, E. A., 2011. Foot-and-mouth disease in pigs: current epidemiological situation and control methods, Transboundary and Emerging Diseases, 59 (Supplement s1), 36–49
Mwiine, F. N., Ayebazibwe, C., Olaho-Mukani, W., Alexandersen S. and Tjørnehøj K., 2010a. Prevalence of Antibodies Against Foot-and-Mouth Disease Virus in Cattle in Kasese and Bushenyi Districts in Uganda, International Journal of Animal and Veterinary Advance, 2(3), 89–96
Mwiine, F. N., Ayebazibwe, C., Olaho-Mukani, W., Alexandersen, S., Balinda, S. N., Masembe, C., Ademun Okurut, A. R., Christensen, L. S., Sørensen, K. J., Tjørnehøj, K., 2010b. Serotype Specificity of Antibodies against Foot-and-Mouth Disease Virus in Cattle in Selected Districts in Uganda, Transboundary and Emerging Diseases, 57(5), 365–374
Pattnaik, B., Suramaniam, S., Sanyal, A., Mohapatra, J. K., Dash, B. B., Ranjan, R. and Rout, M., 2012. Foot-and-mouth disease: Global status and future road map for control and prevention in India, Agriculture Research, 1(2), 132–147
Perry, A.D., 2007, Poverty impacts of foot-and-mouth disease and poverty reduction implications of its control, The Veterinary Record 160, 238–241
Turner, L. R., 2005. Livestock, liberalization and democracy; constraints and opportunities for rural livestock producers in a reforming Uganda, Food and Agricultural Organization, Pro-Poor livestock Resource Initiative, paper 29
UBOS/MAAIF, 2009. The national livestock census: A summary report of the national livestock census, 2008, Ministry of Agriculture Animal Industries and Fisheries and Uganda Bureau of Statistics, 41
Uganda Industrial Research Institute (UIRI), 2011. Brentec Vaccines Limited. Available at: http://www.uiri.org/index.php?option=com_content&view=article&id=127&Itemid=196. Last accessed on 6/10/2012
Windsor, P. A., Freeman, P. G., Abila, R., Benigno, C., Verin, B., Nim, V., and Cameron, A., 2011. Foot-and-mouth disease control and eradication in Bicol surveillance buffer zone of Philippines, Transboundary and Emerging Diseases, 58, 421–433
Acknowledgments
Authors are grateful to the United States Agency for International Development for the funding under the FAR0018479 grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11250-014-0555-z.
Rights and permissions
About this article
Cite this article
Muleme, M., Barigye, R., Khaitsa, M.L. et al. Effectiveness of vaccines and vaccination programs for the control of foot-and-mouth disease in Uganda, 2001–2010. Trop Anim Health Prod 45, 35–43 (2012). https://doi.org/10.1007/s11250-012-0254-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-012-0254-6