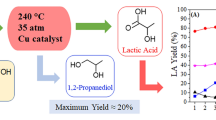
Abstract
Nowadays, many industrial processes depend on fossil resources to obtain high-added-value chemical compounds, bringing with it significant environmental deterioration. Therefore, the implementation of green alternatives considering the use of biomass residues in hydrogen-free catalytic processes is of great relevance. In this work, a set of copper catalysts prepared by impregnating SBA-15 and Zr-SBA-15 (different Si/Zr ratios) supports were catalytically tested in the glycerol dehydration. The copper catalysts were characterized by ICP, SEM-EDS, N2 physisorption, XRD, HRTEM, FT-IR, UV–Vis DRS, XPS, H2-TPR, N2O chemisorption, and TPO analyses. ICP revealed a suitable agreement between nominal and actual copper content in the catalysts. Copper catalysts exhibited textural and structural properties of mesoporous materials which are affected by increasing Zr content. Pyridine FT-IR indicated that copper catalysts with lower Zr content possess the highest values of Lewis acid sites. Higher Zr content makes the copper reduction more difficult, and the dispersion is unfavored. The catalytic system was studied using an 80% wt. aqueous glycerol solution in inert conditions at 220 °C without diffusional limitations according to the Weisz–Prater and Mears criteria. The highest values of specific initial reaction rate and rate constant were obtained in catalysts with zero (Cu/B catalyst, 1.50 × 10−3 molgl/min gcat, 7.79 × 10−4 min−1) and low (Cu/B10 catalyst, 1.53 × 10−3 molgl/min gcat, 8.27 × 10−4 min−1) Zr loading. Hydroxyacetone (HA) and 1,2-propanediol (1,2-PDO) were the main formed products. HA formation reached values greater than 90% selectivity in the first minutes of the reaction, while 1,2-PDO achieved a progressive evolution over time up to 9% selectivity. Initial formation rates of HA (3.29 × 10−4 molHA/min gcat) and 1,2-PDO (2.13 × 10−5 mol1,2−PDO/min gcat) were optimized on the Cu/B10 catalyst containing the lowest Zr load. 1,2-PDO generation in the absence of external hydrogen with a high concentration of glycerol simulating industrial wastes is highlighted. Finally, the Zr load in the copper catalysts modulates the textural, structural, acidic, surface, and reducible properties, which can also be considered as descriptors to understand the catalytic activity together with exposed copper species, stability against copper leaching, sintering, and carbon deposition.















Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Okoye PU, Abdullah AZ, Hameed BH (2017) A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—a byproduct of biodiesel. Renew Sustain Energy Rev 74:387–401
Geilen FMA, Engendahl B, Harwardt A, Marquardt W, Klankermayer J, Leitner W (2010) Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system. Angew Chem Int Ed 49:5510–5514
Kamm B (2007) Production of platform chemicals and synthesis gas from biomass. Angew Chem Int Ed 46:5056–5058
Chatterjee C, Pong F, Sen A (2015) Chemical conversion pathways for carbohydrates. Green Chem 17:40–71
Choi S, Song CW, Shin JH, Lee SY (2015) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 28:223–239
Werpy T, Petersen G (2004) Top value added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas, in, United States,
Garlapati VK, Shankar U, Budhiraja A (2016) Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol Rep 9:9–14
Yang L, He T, Lai C, Chen P, Hou Z (2020) Selective oxidation of glycerol with oxygen in base-free solution over N-doped-carbon-supported Sb@PtSb2 hybrid. Chin J Catal 41:494–502
Xu Y, Nordblad M, Nielsen PM, Brask J, Woodley JM (2011) In situ visualization and effect of glycerol in lipase-catalyzed ethanolysis of rapeseed oil. J Mol Catal B: Enzymatic 72:213–219
Yin H, Zhang C, Yin H, Gao D, Shen L, Wang A (2016) Hydrothermal conversion of glycerol to lactic acid catalyzed by Cu/hydroxyapatite, Cu/MgO, and Cu/ZrO2 and reaction kinetics. Chem Eng J 288:332–343
Izquierdo JF, Montiel M, Palés I, Outón PR, Galán M, Jutglar L, Villarrubia M, Izquierdo M, Hermo MP, Ariza X (2012) Fuel additives from glycerol etherification with light olefins: state of the art. Renew Sustain Energy Rev 16:6717–6724
Ozbay N, Oktar N, Dogu G, Dogu T (2013) Activity comparison of different solid acid catalysts in etherification of glycerol with tert-butyl alcohol in Flow and batch reactors. Top Catal 56:1790–1803
Martin A, Richter M (2011) Oligomerization of glycerol—a critical review. Eur J Lipid Sci Technol 113:100–117
Talebian-Kiakalaieh A, Amin NAS, Hezaveh H (2014) Glycerol for renewable acrolein production by catalytic dehydration. Renew Sustain Energy Rev 40:28–59
Crotti C, Kašpar J, Farnetti E (2010) Dehydrogenation of glycerol to dihydroxyacetone catalyzed by iridium complexes with P–N ligands. Green Chem 12:1295–1300
Dodekatos G, Schünemann S, Tüysüz H (2018) Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal 8:6301–6333
Teng WK, Ngoh GC, Yusoff R, Aroua MK (2014) A review on the performance of glycerol carbonate production via catalytic transesterification: effects of influencing parameters. Energy Conv Manag 88:484–497
Kolena J, Skuhrovcová L, Kocík J, Šafář J, Kupčík J (2018) Catalyst for selective hydrogenolysis of glycerol, prepared from hydrotalcite-like structures. Top Catal 61:1746–1756
Liu Y, Pasupulety N, Gunda K, Rempel GL, Ng FTT (2014) Glycerol hydrogenolysis to 1,2-propanediol by Cu/ZnO/Al2O3 catalysts. Top Catal 57:1454–1462
Ebshish A, Yaakob Z, Taufiq-Yap YH, Bshish A, Tasirin SM (2012) Review of hydrogen production via glycerol reforming. J Power Energy 226:1060–1075
Schwengber CA, Alves HJ, Schaffner RA, da Silva FA, Sequinel R, Bach VR, Ferracin RJ (2016) Overview of glycerol reforming for hydrogen production. Renew Sustain Energy Rev 58:259–266
Charisiou ND, Papageridis KN, Siakavelas G, Tzounis L, Kousi K, Baker MA, Hinder SJ, Sebastian V, Polychronopoulou K, Goula MA (2017) Glycerol steam reforming for hydrogen production over nickel supported on alumina, zirconia and silica catalysts. Top Catal 60:1226–1250
Inns DR, Mayer AJ, Skukauskas V, Davies TE, Callison J, Kondrat SA (2021) Evaluating the activity and stability of perovskite LaMO3-based pt catalysts in the aqueous phase reforming of glycerol. Top Catal 64:992–1009
Talebian-Kiakalaieh A, Amin NAS, Najaafi N, Tarighi S (2018) A review on the catalytic acetalization of bio-renewable glycerol to fuel additives. Front Chem. https://doi.org/10.3389/fchem.2018.00573
Trifoi AR, Agachi PŞ, Pap T (2016) Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew Sustain Energy Rev 62:804–814
Suganuma S, Hisazumi T, Taruya K, Tsuji E, Katada N (2017) Influence of Acidic Property on Catalytic Activity and selectivity in dehydration of glycerol. ChemistrySelect 2:5524–5531
Neto ASB, Oliveira AC, Filho JM, Amadeo N, Dieuzeide ML, de Sousa FF, Oliveira AC (2017) Characterizations of nanostructured nickel aluminates as catalysts for conversion of glycerol: influence of the preparation methods. Adv Powder Technol 28:131–138
Talebian-Kiakalaieh A, Amin NAS (2017) Thermo-kinetic and diffusion studies of glycerol dehydration to acrolein using HSiW-γ-Al2O3 supported ZrO2 solid acid catalyst. Renew Energy 114:794–804
Mane RB, Yamaguchi A, Malawadkar A, Shirai M, Rode CV (2013) Active sites in modified copper catalysts for selective liquid phase dehydration of aqueous glycerol to acetol. RSC Adv 3:16499–16508
de Araújo ML, Mandelli D, Kozlov YN, Carvalho WA (2016) Shul’pin, oxidation of hydroxyacetone (acetol) with hydrogen peroxide in acetonitrile solution catalyzed by iron(III) chloride. J Mol Catal A: Chem 422:103–114
Célerier S, Morisset S, Batonneau-Gener I, Belin T, Younes K, Batiot-Dupeyrat C (2018) Glycerol dehydration to hydroxyacetone in gas phase over copper supported on magnesium oxide (hydroxide) fluoride catalysts. Appl Catal A 557:135–144
Braga TP, Essayem N, Valentini A (2016) Non-crystalline copper oxide highly dispersed on mesoporous alumina synthesis, characterization and catalytic activity in glycerol conversion to acetol. Quím Nova 39:691–696
García-Sancho C, Cecilia JA, Mérida-Robles JM, Santamaría González J, Moreno-Tost R, Infantes-Molina A, Maireles-Torres P (2018) Effect of the treatment with H3PO4 on the catalytic activity of Nb2O5 supported on Zr-doped mesoporous silica catalyst. Case study: glycerol dehydration. Appl Catal B 221:158–168
Possato LG, Diniz RN, Garetto T, Pulcinelli SH, Santilli CV, Martins L (2013) A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J Catal 300:102–112
Bezerra FA, Altino HON, Soares RR (2019) Oxidative dehydration of glycerol over molybdenum- and vanadium-based catalysts. J Braz Chem Soc 30:1025–1033
Deleplanque J, Dubois JL, Devaux JF, Ueda W (2010) Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catal Today 157:351–358
Viswanadham B, Srikanth A, Chary KVR (2014) Characterization and reactivity of 11-molybdo-1-vanadophosphoric acid catalyst supported on zirconia for dehydration of glycerol to acrolein. J Chem Sci 126:445–454
Basu S, Sen AK, Mukherjee M (2019) Synthesis and performance evaluation of silica-supported copper chromite catalyst for glycerol dehydration to acetol. J Chem Sci 131:82
Ma T, Ding J, Shao R, Xu W, Yun Z (2017) Dehydration of glycerol to acrolein over Wells–Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem Eng J 316:797–806
Chimentão RJ, Miranda BC, Ruiz D, Gispert-Guirado F, Medina F, Llorca J, Santos JBO (2020) Catalytic performance of zinc-supported copper and nickel catalysts in the glycerol hydrogenolysis. J Energy Chem 42:185–194
Yun D, Yun YS, Kim TY, Park H, Lee JM, Han JW, Yi J (2016) Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate. J Catal 341:33–43
Ma T, Yun Z, Xu W, Chen L, Li L, Ding J, Shao R (2016) Pd-H3PW12O40/Zr-MCM-41: an efficient catalyst for the sustainable dehydration of glycerol to acrolein. Chem Eng J 294:343–352
Pala Rosas I, Contreras JL, Salmones J, Tapia C, Zeifert B, Navarrete J, Vázquez T, García DC (2017) Catalytic dehydration of glycerol to acrolein over a catalyst of Pd/LaY zeolite and comparison with the chemical equilibrium. Catalysts. https://doi.org/10.3390/catal7030073
Volkov SV, Khar’kova LB, Baranets SA, Yanko OG, Strizhak PE, Kosmambetova GR, Gritsenko VI (2016) Catalytic properties of RhSe2/Ga/H-ZSM-5 system in the reaction of glycerol dehydration in the gas phase. Russ J Appl Chem 89:233–237
Gu Y, Liu S, Li C, Cui Q (2013) Selective conversion of glycerol to acrolein over supported nickel sulfate catalysts. J Catal 301:93–102
Miranda BC, Chimentão RJ, Santos JBO, Gispert-Guirado F, Llorca J, Medina F, Bonillo FL, Sueiras JE (2014) Conversion of glycerol over 10%Ni/γ-Al2O3 catalyst. Appl Catal B 147:464–480
Carvalho DC, Pinheiro LG, Campos A, Millet ERC, de Sousa FF, Filho JM, Saraiva GD, Filho ECdS, Fonseca MG, Oliveira AC (2014) Characterization and catalytic performances of copper and cobalt-exchanged hydroxyapatite in glycerol conversion for 1-hydroxyacetone production. Appl Catal A 471:39–49
Yue C-J, Gan M-M, Gu L-P, Zhuang Y-F (2014) In situ synthesized nano-copper over ZSM-5 for the catalytic dehydration of glycerol under mild conditions. J Taiwan Inst Chem Eng 45:1443–1448
Mitta H, Seelam PK, Ojala S, Keiski RL, Balla P (2018) Tuning Y-zeolite based catalyst with copper for enhanced activity and selectivity in vapor phase hydrogenolysis of glycerol to 1,2-propanediol. Appl Catal A 550:308–319
Huang L, Zhu Y, Zheng H, Ding G, Li Y (2009) Direct conversion of glycerol into 1,3-propanediol over Cu-H4SiW12O40/SiO2 in vapor phase. Catal Lett 131:312–320
Huang Z, Cui F, Kang H, Chen J, Zhang X, Xia C (2008) Highly dispersed silica-supported copper nanoparticles prepared by precipitation—gel method: a simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem Mater 20:5090–5099
Suthagar K, Shanthi K, Selvam P (2018) Hydrogenolysis of glycerol over silica-supported copper-nanocatalyst: effect of precipitating-agent and copper metal-loading. Mol Catal 458:307–316
Gabrysch T, Peng B, Bunea S, Dyker G, Muhler M (2018) The role of metallic copper in the selective hydrodeoxygenation of glycerol to 1,2-propanediol over Cu/ZrO2. ChemCatChem 10:1344–1350
Feng Y, Yin H, Wang A, Shen L, Yu L, Jiang T (2011) Gas phase hydrogenolysis of glycerol catalyzed by Cu/ZnO/MOx (MOx = Al2O3, TiO2, and ZrO2) catalysts. Chem Eng J 168:403–412
Dar BA, Dadhwal S, Singh G, Garg P, Sharma P, Singh B (2013) Vapour phase conversion of glycerol to acrolein over supported copper. Arab J Sci Eng 38:37–40
Zheng J, Zhu W, Ma C, Hou Y, Zhang W, Wang Z (2010) Hydrogenolysis of glycerol to 1,2-propanediol on the high dispersed SBA-15 supported copper catalyst prepared by the ion-exchange method. Reaction Kinet Mech Catal 99:455–462
Dieuzeide ML, de Urtiaga R, Jobbagy M, Amadeo N (2017) Vapor phase hydrogenolysis of glycerol to 1,2-propanediol at atmospheric pressure over copper catalysts supported on mesoporous alumina. Catal Today 296:19–25
Braga TP, Essayem N, Prakash S, Valentini A (2016) Gas-phase conversion of glycerol to acetol: influence of support acidity on the catalytic stability and copper surface properties on the activity. J Braz Chem Soc 27:2361–2371
Zhang H, Hu Z, Huang L, Zhang H, Song K, Wang L, Shi Z, Ma J, Zhuang Y, Shen W, Zhang Y, Xu H, Tang Y (2015) Dehydration of glycerol to acrolein over hierarchical ZSM-5 zeolites: effects of mesoporosity and acidity. ACS Catal 5:2548–2558
Ferreira P, Fonseca IM, Ramos AM, Vital J, Castanheiro JE (2010) Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl Catal B 98:94–99
Feliczak-Guzik A, Nowak I (2019) Application of glycerol to synthesis of solvo-surfactants by using mesoporous materials containing niobium. Microporous Mesoporous Mater 277:301–308
Martin A, Armbruster U, Atia H (2012) Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur J Lipid Sci Technol 114:10–23
Chen L-F, Guo P-J, Zhu L-J, Qiao M-H, Shen W, Xu H-L, Fan K-N (2009) Preparation of Cu/SBA-15 catalysts by different methods for the hydrogenolysis of dimethyl maleate to 1,4-butanediol. Appl Catal A 356:129–136
Liu P, Zhou C-Y, Xiang S, Che C-M (2010) Highly efficient oxidative carbon–carbon coupling with SBA-15-support iron terpyridine catalyst. Chem Commun 46:2739–2741
Huirache-Acuña R, Nava R, Peza-Ledesma CL, Lara-Romero J, Alonso-Núez G, Pawelec B, Rivera-Muñoz EM (2013) SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts—review. Materials. https://doi.org/10.3390/ma6094139
Wang Y, Noguchi M, Takahashi Y, Ohtsuka Y (2001) Synthesis of SBA-15 with different pore sizes and the utilization as supports of high loading of cobalt catalysts. Catal Today 68:3–9
Carrero A, Vizcaíno AJ, Calles JA, García-Moreno L (2017) Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with zr, ce and La. J Energy Chem 26:42–48
Chary KVR, Ramesh K, Vidyasagar G, Venkat Rao V (2003) Vapour phase alkylation of phenol with methanol over vanadium oxide supported on zirconia. J Mol Catal A: Chem 198:195–204
Chary KVR, Ramesh K, Naresh D, Rao PVR, Rao AR, Rao VV (2009) The effect of zirconia polymorphs on the structure and catalytic properties of V2O5/ZrO2 catalysts. Catal Today 141:187–194
Colmenares-Zerpa J, Chimentão RJ, Gispert-Guirado F, Peixoto AF, Llorca J (2021) Preparation of SBA-15 and Zr-SBA-15 materials by direct-synthesis and pH-adjustment methods. Mater Lett 301:130326
Colmenares-Zerpa J, Gajardo J, Peixoto AF, Silva DSA, Silva JA, Gispert-Guirado F, Llorca J, Urquieta-Gonzalez EA, Santos JBO, Chimentão RJ (2022) High zirconium loads in Zr-SBA-15 mesoporous materials prepared by direct-synthesis and pH-adjusting approaches. J Solid State Chem 312:123296
Katryniok B, Paul S, Bellière-Baca V, Rey P, Dumeignil F (2010) Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem 12:2079–2098
Sun D, Yamada Y, Sato S, Ueda W (2016) Glycerol hydrogenolysis into useful C3 chemicals. Appl Catal B 193:75–92
Wu S, Han Y, Zou Y-C, Song J-W, Zhao L, Di Y, Liu S-Z, Xiao F-S (2004) Synthesis of heteroatom substituted SBA-15 by the pH-adjusting. Method Chem Mater 16:486–492
Tamura M, Shimizu K-I, Satsuma A (2012) Comprehensive IR study on acid/base properties of metal oxides. Appl Catal A: General 433–434:135–145
Yang L, Kruse B (2004) Revised kubelka–Munk theory. I. Theory and application. J Opt Soc Am a 21:1933–1941
Bernadette P-I, Daniel D, Claude C (2001) Bibliographical review for reflectance of diffusing media. Opt Eng 40:1082–1092
Wood DL, Tauc J (1972) Weak absorption tails in amorphous semiconductors. Phys Rev B 5:3144–3151
Zavrazhnov SA, Esipovich AL, Zlobin SY, Belousov AS, Vorotyntsev AV (2019) Mechanism analysis and kinetic modelling of Cu NPs catalysed glycerol conversion into lactic acid. Catalysts. https://doi.org/10.3390/catal9030231
Fogler HS (2016) Elements of chemical reaction engineering, 5th edn. Prentice Hall, Philadelphia
Yfanti VL, Ipsakis D, Lemonidou AA (2018) Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. Reaction Chem Eng 3:559–571
Thommes M (2010) Physical adsorption characterization of nanoporous materials. Chem Ing Tech 82:1059–1073
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Thunyaratchatanon C, Luengnaruemitchai A, Chaisuwan T, Chollacoop N, Chen S-Y, Yoshimura Y (2017) Synthesis and characterization of Zr incorporation into highly ordered mesostructured SBA-15 material and its performance for CO2 adsorption. Microporous Mesoporous Mater 253:18–28
Qiang T, Zhao J, Li J (2018) Direct synthesis of homogeneous Zr-doped SBA-15 mesoporous silica via masking zirconium sulfate. Microporous Mesoporous Mater 257:162–174
Chaudhary V, Sharma S (2017) An overview of ordered mesoporous material SBA-15: synthesis, functionalization and application in oxidation reactions. J Porous Mater 24:741–749
Rouquerol J, Llewellyn P, Rouquerol F (2007) Is the bet equation applicable to microporous adsorbents? In: Llewellyn PL, Rodriquez-Reinoso F, Rouqerol J, Seaton N (eds) Studies in surface science and catalysis. Elsevier, Amsterdam, pp 49–56
Fitting, Optimizing BET (2014) Surface area, Micromeritics instrument corporation
Wang L, Kong A, Chen B, Ding H, Shan Y, He M (2005) Direct synthesis, characterization of Cu-SBA-15 and its high catalytic activity in hydroxylation of phenol by H2O2. J Mol Catal A: Chem 230:143–150
Miao K-K, Luo X-l, Wang W, Guo J-l, Guo S-F, Cao F-J, Hu Y-Q, Chang P-M, Feng G-D (2019) One-step synthesis of Cu–SBA-15 under neutral condition and its oxidation catalytic performance. Microporous Mesoporous Mater 289:109640
Veisi H, Karmakar B, Tamoradi T, Hemmati S, Hekmati M, Hamelian M (2021) Biosynthesis of CuO nanoparticles using aqueous extract of herbal tea (Stachys lavandulifolia) flowers and evaluation of its catalytic activity. Sci Rep 11:1983
Butte SM, Waghuley SA (2020) Optical properties of Cu2O and CuO, AIP conference proceedings, 2220, p. 020093
Siddiqui H, Parra MR, Pandey P, Qureshi MS, Haque FZ (2020) Utility of copper oxide nanoparticles (CuO–NPs) as efficient electron donor material in bulk-heterojunction solar cells with enhanced power conversion efficiency. J Sci: Adv Mater Devices 5:104–110
Hemmann F, Agirrezabal-Telleria I, Jaeger C, Kemnitz E (2015) Quantification of acidic sites of nanoscopic hydroxylated magnesium fluorides by FTIR and 15 N MAS NMR spectroscopy. RSC Adv 5:89659–89668
Sekar K, Chuaicham C, Balijapalli U, Li W, Wilson K, Lee AF, Sasaki K (2021) Surfactant- and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl Catal B 284:119741
Zhao J, Chen H, Xu J, Shen J (2013) Effect of surface acidic and basic properties of the supported nickel catalysts on the hydrogenation of pyridine to piperidine. J Phys Chem C 117:10573–10580
Lin W, Herzing AA, Kiely CJ, Wachs IE (2008) Probing metal—support interactions under oxidizing and reducing conditions: in situ Raman and infrared spectroscopic and scanning transmission electron microscopic—X-ray energy-dispersive spectroscopic investigation of supported platinum catalysts. J Phys Chem C 112:5942–5951
Sato AG, Volanti DP, Meira DM, Damyanova S, Longo E, Bueno JMC (2013) Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J Catal 307:1–17
Wu G-S, Mao D-S, Lu G-Z, Cao Y, Fan K-N (2009) The role of the promoters in Cu based catalysts for methanol steam reforming. Catal Lett 130:177–184
Zhang W, Yao Y, Xie S, Gubsch K, Yang Y, Lan X, Lin H (2021) Synergistic interaction between Cu and ZrO2 promotes ethyl formate hydrogenation to produce methanol. Catal Today 374:53–60
Bosman HJM, Pijpers AP, Jaspers AWMA (1996) An X-ray photoelectron spectroscopy study of the acidity of SiO2–ZrO2Mixed oxides. J Catal 161:551–559
Tang Q, Xu H, Zheng Y, Wang J, Li H, Zhang J (2012) Catalytic dehydration of methanol to dimethyl ether over micro–mesoporous ZSM-5/MCM-41 composite molecular sieves. Appl Catal A. https://doi.org/10.1016/j.apcata.2011.10.039
Tang Y, Zong E, Wan H, Xu Z, Zheng S, Zhu D (2012) Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal. Microporous Mesoporous Mater 155:192–200
Yang G, Wang L, Jiang H (2020) Zr-incorporating SBA-15 for conversion of the ethanol–acetaldehyde mixture to butadiene. Reaction Chem Eng 5:1833–1844
Sanjini NS, Velmathi S (2014) Iron impregnated SBA-15, a mild and efficient catalyst for the catalytic hydride transfer reduction of aromatic nitro compounds. RSC Adv 4:15381–15388
Newalkar BL, Olanrewaju J, Komarneni S (2001) Microwave-hydrothermal synthesis and characterization of zirconium substituted SBA-15 mesoporous silica. J Phys Chem B 105:8356–8360
Emeline A, Kataeva GV, Litke AS, Rudakova AV, Ryabchuk VK, Serpone N (1998) Spectroscopic and photoluminescence studies of a wide band gap insulating material: powdered and colloidal ZrO2 sols. Langmuir 14:5011–5022
Pestryakov AN, Petranovskii VP, Kryazhov A, Ozhereliev O, Pfänder N, Knop-Gericke A (2004) Study of copper nanoparticles formation on supports of different nature by UV–Vis diffuse reflectance spectroscopy. Chem Phys Lett 385:173–176
Chanquía CM, Sapag K, Rodríguez-Castellón E, Herrero ER, Eimer GA (2010) Nature and location of copper nanospecies in mesoporous molecular sieves. J Phys Chem C 114:1481–1490
Tsoncheva T, Genova I, Dimitrov M, Sarcadi-Priboczki E, Venezia AM, Kovacheva D, Scotti N, Dal Santo V (2015) Nanostructured copper–zirconia composites as catalysts for methanol decomposition. Appl Catal B 165:599–610
Sharma PK, Cortes MALRM, Hamilton JWJ, Han Y, Byrne JA, Nolan M (2019) Surface modification of TiO2 with copper clusters for band gap narrowing. Catal Today. https://doi.org/10.1016/j.cattod.2017.12.002
Mukherjee A, Ghosh P, Aboud AA, Mitra P (2016) Influence of copper incorporation in CdS: structural and morphological studies. Mater Chem Phys 184:101–109
Fahmi Khairol N, Sapawe N, Danish M (2020) Study the band gap properties of copper incorporated onto eggshell using UV–Vis diffuse reflectance spectroscopy. Mater Today: Proc 31:237–240
Chauhan D, Satsangi V, Dass S, Shrivastav R (2006) Preparation and characterization of nanostructured CuO thin films for photoelectrochemical splitting of water. Bull Mater Sci 29:709–716
Tahir D, Tougaard S (2012) Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J Phys: Condens Matter 24:175002
S JRR, X PCMAD (2022) The influence of Cu doped ZrO2 catalyst for the modification of the rate of a photoreaction and forming microorganism resistance. J Water Environ Nanatechnol 7:351–362
Vargas-Hernández D, Rubio-Caballero JM, Santamaría-González J, Moreno-Tost R, Mérida-Robles JM, Pérez-Cruz MA, Jiménez-López A, Hernández-Huesca R, Maireles-Torres P (2014) Furfuryl alcohol from furfural hydrogenation over copper supported on SBA-15 silica catalysts. J Mol Catal A: Chem. https://doi.org/10.1016/j.molcata.2013.11.034
Harisekhar M, Pavan Kumar V, Shanthi Priya S, Chary KVR (2015) Vapour phase hydrogenolysis of glycerol to propanediols over Cu/SBA-15 catalysts. J Chem Technol Biotechnol 90:1906–1917
Wu Y, Tan L, Zhang T, Xie H, Yang G, Tsubaki N, Chen J (2019) Effect of preparation method on ZrO2-based catalysts performance for isobutanol synthesis from syngas. Catalysts. https://doi.org/10.3390/catal9090752
Wei L, Zeng C-Y, Xie H-J, Wu Y-Q (2021) Study on the formation of 2-pentanone from ethanol over K-CuZrO2 catalysts. J Fuel Chem Technol 49:80–87
Espinós JP, Morales J, Barranco A, Caballero A, Holgado JP, González-Elipe AR (2002) Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts. J Phys Chem B 106:6921–6929
Rodriguez JA, Kim JY, Hanson JC, Pérez M, Frenkel AI (2003) Reduction of CuO in H2: in situ time-resolved XRD studies. Catal Lett 85:247–254
Colmenares-Zerpa J, Gajardo J, González G, Fierro JLG, Peixoto AF, Junkaew A, Suthirakun S, Santos JBO, Picinini M, Urquieta-Gonzalez EA, Hirunsit P, Chimentão RJ (2023) Catalytic valorization of glycerol in the absence of external hydrogen: effect of the Cu/ZrO2 catalyst mass and solvent. Catal Today 423:114275
Trunschke A, Hoang DL, Lieske H (1995) In situ FTIR studies of high-temperature adsorption of hydrogen on zirconia. J Chem Soc Faraday Trans 91:4441–4444
Pandya R, Mane R, Rode C (2022) Influence of catalyst reduction temperature on autogenous glycerol hydrogenolysis over NiAl catalyst. Asian J Org Chem 11:e202100704
De Vrieze JE, Thybaut JW, Saeys M (2018) Role of surface hydroxyl species in copper-catalyzed hydrogenation of ketones. ACS Catal 8:7539–7548
Liu J, Li Y, Zhang J, He D (2016) Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl Catal A 513:9–18
Atia H, Armbruster U, Martin A (2011) Influence of alkaline metal on performance of supported silicotungstic acid catalysts in glycerol dehydration towards acrolein. Appl Catal A 393:331–339
Chai S-H, Wang H-P, Liang Y, Xu B-Q (2007) Sustainable production of acrolein: investigation of solid acid–base catalysts for gas-phase dehydration of glycerol. Green Chem 9:1130–1136
Ain QT, Haq SH, Alshammari A, Al-Mutlaq MA, Anjum MN (2019) The systemic effect of PEG-nGO-induced oxidative stress in vivo in a rodent model. Beilstein J Nanotechnol 10:901–911
Wawrzetz A, Peng B, Hrabar A, Jentys A, Lemonidou AA, Lercher JA (2010) Towards understanding the bifunctional hydrodeoxygenation and aqueous phase reforming of glycerol. J Catal 269:411–420
Roy D, Subramaniam B, Chaudhari RV (2010) Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal Today 156:31–37
Wu G-S, Wang L-C, Liu Y-M, Cao Y, Dai W-L, He H-Y, Fan K-N (2006) Implication of the role of oxygen anions and oxygen vacancies for methanol decomposition over zirconia supported copper catalysts. Appl Surf Sci 253:974–982
Barton DG, Shtein M, Wilson RD, Soled SL, Iglesia E (1999) Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J Phy Chem B 103:630–640
Acknowledgements
RJC, JG, and JCZ acknowledge the funding provided by Fondecyt with the projects N° 1220355 and N° 1180243. JCZ appreciates the funding provided by the doctoral scholarship No 21201413 awarded by ANID and the internationalization program “UCO 1866”. A.F.P thanks Fundação para a Ciência e a Tecnologia (FCT/MCTES) funding through the projects UIDB/50006/2020 and UIDP/50006/2020. A.F.P. also thanks the work contract under the Scientific Employment Stimulus (2020.01614.CEECIND/CP1596/CT0007). JL is a Serra Húnter Fellow and is grateful to the ICREA Academia program and projects MICINN/FEDER PID2021-124572OB-C31 and GC 2021 SGR 01061.
Author information
Authors and Affiliations
Contributions
JC-Z: conceptualization, methodology, formal analysis and investigation, software, writing—original draft preparation, writing—review and editing, JG: methodology, formal analysis and investigation, AFP: resources, software, writing—review and editing, FG-G: resources, software, writing—review and editing, JL: resources, software, writing—review and editing, EAU-G: resources, writing—review and editing, DSAS: methodology, formal analysis and investigation, JBOS: resources, writing—review and editing, RJC: conceptualization, methodology, software, writing—original draft preparation, writing—review and editing, funding acquisition, resources, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
In honorary memory of Professor Eduardo José Leonardo Delgado Ramírez.
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Colmenares-Zerpa, J., Gajardo, J., Peixoto, A.F. et al. Turning Glycerol to Value-Added Chemicals in the Absence of External Hydrogen over Copper Catalysts Supported on SBA-15-Type Materials Containing Zirconium. Top Catal 67, 422–453 (2024). https://doi.org/10.1007/s11244-023-01879-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01879-4