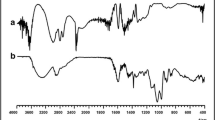
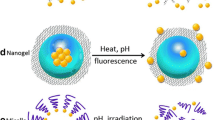
Abstract
The efficient biodistribution and controlled release of medications or genes at specific site are made possible by the use of stimuli-responsive or intelligent polymers. Pathologically challenged tissue has fundamental qualities that are very different from those of typical, healthy cells. These characteristics have been useful in creating endostimuli-responsive nanocarriers for the efficient delivery of drug cargoes. The site-specific release of medications delivered by nanocarriers can take advantage of the tumour microenvironment's increased temperature and acidic pH. Capecitabine, is enzymatically catalysed to 5-FU, the active compound by sequential enzymatic steps with the last step catalyzed by the tumor-associated angiogenic factor thymidine phosphorylase which is more abundantly expressed in many types of human tumours than in healthy tissues catalyses. Thus, the site specific delivery of capacitabine to tumor microenvironment enhance its catalytic activity and increase the selectivity of 5-FU for tumor cells and decrease plasma levels of 5-FU. The goal of this research was to synthesize the co-polymer chitosan-g- poly (N-isopropylacrylamide) (CS-g-PNIPAm) and evaluate it as a dual responsive carrier for targeted Capecitabine delivery. For this, the co-polymer was synthesized, its responsiveness was optimized to tumour microenvironment conditions of pH and temperature. The Capecitabine was subsequently encapsulated in the synthesised co-polymer, and the physicochemical properties of the produced nanoparticles were assessed. When comparing the physiological pH and temperature to acidic pH (6.8) and higher temperature (39 °C), the in vitro stimuli driven drug release investigation found that the percent drug release was greater at acidic pH (6.8) and higher temperature (39 °C). MTT assay as well asfluorescence microscopic study demonstrated significantly increased drug release in tumor microenvironment while showed minimal effect at physiological conditions. In conclusion, the synthesizedco-polymer appear to be an an efficient dual pH and temperature responsive carrier for targeted delivery of anti-cancer drug Capecitabine to enhance its catalytic activity. Higher levels of FU are thus produced within tumours with minimal exposure of healthy tissue to FU.
Graphical Abstract










Similar content being viewed by others
Reference:s
American Association for Cancer Research (2013) AACR cancer progress report. Clin Cancer Res 19:S1–S88
Mathew TV, Baby B, Wang K, Lei F, Chen Q, Huang B, Mathew A (2020) Trends in colorectal cancer incidence in India. Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.e16084
Patil PS, Saklani A, Gambhire P, Mehta S, Engineer R, De’Souza A, Bal M, (2017) Colorectal cancer in India: an audit from a tertiary center in a low prevalence area. Indian J Surg Oncol 8:484–490
American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020
IshikawaT UM, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H (1998) Tumor selective delivery of 5-fluorouracil by capecitabine a new oral fluoropyrimidine carbamate in human cancer xenografts. Biochem Pharmacol 55(7):1091–1097
Miwa M, Ura M, Nishida M, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate capecitabine which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients Cancer. Chemo ther Pharmacol 45:291
Moghaddam A, Zhang HT, Fan TP, Hu DE, Lees VC, Turley H, Fox SB, Gatter KC, Harris AL, Bicknell R (1992) Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci USA 92:998–1002
Arima J, Imazono Y, Takebayashi Y, Nishiyama K, Shirahama T, Akiba S, Furukawa T, Akiyama S, Ohi Y (2000) Expression of thymidine phosphorylase as an indicator of poor prognosis for patients with transitional cell carcinoma of the bladder. Cancer 88:1131–1138
O’Brien TS, Fox SB, Dickinson AJ, Turley H, Westwood M, Moghaddam A, Gatter KC, Bicknell R, Harris AL (1996) Expression of the angiogenic factor thymidine phosphorylase/ platelet-derived endothelial cell growth factor in primary bladder cancers. Cancer Res 56:4799–4804
Yoshikawa T, Suzuki K, Kobayashi O, Sairenji M, Motohashi H, Tsuburaya A, Nakamura Y, Shimizu A, Yanoma S, Noguchi Y (1999) Thymidine phosphorylase/platelet-derived endothelial cell growth factor is upregulated in advanced solid types of gastric cancer. Br J Cancer 79:1145–1150
Takebayashi Y, Miyadera K, Akiyama S, Hokita S, Yamada K, Akiba S, Yamada Y, Sumizawa T, Aikou T (1996) Expression of thymidine phosphorylase in human gastric carcinoma. Jpn J Cancer Res 87:288–295
Takebayashi Y, Yamada K, Miyadera K, Sumizawa T, Furukawa T, Kinoshita F, Aoki D, Okumura H, Yamada Y, Akiyama S, Aikou T (1996) The activity and expression of thymidine phosphorylase in human solid tumours. Eur J Cancer 32A:1227–1232
O’Byrne KJ, Koukourakis MI, Giatromanolaki A, Cox G, Turley H, Steward WP, Gatter K, Harris AL (2000) Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer 82:1427–1432
Takebayashi Y, Natsugoe S, Baba M, Akiba S, Fukumoto T, Miyadera K, Yamada Y, Takao S, Akiyama S, Aikou T (1999) Thymidine phosphorylase in human esophageal squamous cell carcinoma. Cancer 85:282–289
Fujimoto J, Sakaguchi H, Hirose R, Wen H, Tamaya T (1999) Clinical implication of expression of platelet-derived endothelial cell growth factor (PD-ECGF) in metastatic lesions of uterine cervical cancers. Cancer Res 59:3041–3044
Yamamoto A, Dhar DK, El Assal ON, Igarashi M, Tabara H, Nagasue N (1998) Thymidine phosphorylase (platelet-derived endothelial cell growth factor), microvessel density and clinical outcome in hepatocellular carcinoma. J Hepatol 29:290–299
Bronckaers A, Gago F, Balzarini J, Liekens S (2009) The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med Res Rev 29(6):903–953
Vincent MD, Breadner D, Soulieres D, Kerr IG, Sanatani M, Kocha W, Welch SA (2017) Phase II trial of capecitabine plus erlotinib versus capecitabine alone in patients with advanced colorectal cancer. Future Oncol 13:777–786
Sun SB, Liu P, Shao FM, Miao QL (2015) Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int J Clin Exp 8:19670
Sinha VR, Bhinge JR (2007) Platform technologies in colon delivery. Pharma Buzz 1:13–15
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–146
Cui W, Lu X, Cui K, Niu L, Wei Y, Lu Q (2012) Dual-responsive controlled drug delivery based on ionically assembled nanoparticles. Langmuir 28:9413–9420
Oerlemans C, Bult W, Bos M, Storm G, Nijsen J, Hennink WE (2010) Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res 27:2569–2589
Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, Quan Z (2017) pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf 154:287–296
Rakhshaei R, Namazi H, Hamishehkar H, Rahimi M (2020) Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int J Biol Macromol 150:1121–1129
Song Q, Jia J, Niu X, Zheng C, Zhao H, Sun L, Zhang Y (2019) An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale 11:15958–15970
Woraphatphadung T, Sajomsang W, Rojanarata T, Ngawhirunpat T, Tonglairoum P, Opanasopit P (2018) Development of chitosan-based pH-sensitive polymeric micelles containing curcumin for colon-targeted drug delivery. AAPS Pharm Sci Tech 19:991–1000
De V, Milano F, Mancini E, Comparelli R, Giotta L, Nacci A, Catucci L (2018) Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 23:739
Bawa P, Pillay V, Du ChoonaraYE, LC (2009) Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater 4:022001
Motamarry A, Negussie H, Rossmann C, Small J, Wolfe M, Wood J, Haemmerich D (2019) Real-time fluorescence imaging for visualization and drug uptake prediction during drug delivery by thermosensitive liposomes. Int J Hyperth 36:816–825
Nabavizadeh F, Fanaei H, Imani A, Vahedian J, AsadiAmoli F, Ghorbi J, Sohanaki H, Mohammadi SM, Golchoobian R (2016) evaluation of nanocarrier targeted drug delivery of capecitabine-PAMAM dendrimer complex in a mice colorectal cancer model. Acta Med Iran 54:485–493
Hossein A, Alizadeh N (2022) Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv 12(8):4681–4691
Nazari-Vanani R, Karimian K, Azarpira N, Heli H (2019) Capecitabine-loaded nanoniosomes and evaluation of anticancer efficacy. Artif Cells Nanomed Biotechnol 47:420–426
Honmane SM, Chimane SM, Bandgar SA, Patil SS (2020) Development and optimization of capecitabine loaded nanoliposomal system for cancer delivery. Indian J of Pharmaceutical Education and Research 54:376–384
Rajasree PH, Willi P, Chandra PS, Riyaz Ali MO, Umme H, Atul S (2018) Eudragit encapsulated cationic poly (lactic-co-glycolic acid) nanoparticles in targeted delivery of capecitabine for augmented colon carcinoma therapy. J Drug Deliv Sci Technol 46:302–311
Borys N, Dewhirst W (2021) Drug development of lyso-thermosensitive liposomal doxorubicin: combining hyperthermia and thermosensitive drug delivery. Adv Drug Deliv Rev 178:113985
Shen B, Ma Y, Yu S, Ji C (2016) Smart multifunctional magnetic nanoparticle-based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. ACS Appl Mater Interfaces 8:24502–24508
Tamarov K, Xu W, Osminkina L, Zinovyev S, Soininen P, Kudryavtsev A, Lehto VP (2016) Temperature responsive porous silicon nanoparticles for cancer therapy–spatiotemporal triggering through infrared and radiofrequency electromagnetic heating. J Control Release 241:220–228
Li SK, D’Emanuele A (2003) Effect of thermal cycling on the properties of thermoresponsive poly (N-isopropylacrylamide) hydrogels. Int J pharm 267(1–2):27–34
Chen X, Ding X, Zheng Z, Peng Y (2006) Thermosensitive cross-linked polymer vesicles for controlled release system. New J Chem 30(4):577–582
Qin S, Geng Y, Discher DE, Yang S (2006) Temperature-controlled assembly and release from polymer vesicles of poly (ethylene oxide)-block-poly (N-isopropylacrylamide). Adv Mater 18(21):2905–2909
Satapathy SS, Bhol P, Chakkarambath A, Mohanta J, Samantaray K, Bhat SK, Panda SK, Mohanty PS, Si S (2017) Thermo-responsive PNIPAM-metal hybrids: an efficient nanocatalyst for the reduction of 4-nitrophenol. Appl Sur Sci 420:753–763
Wang Y, Zhu L, Zhang H, Huang H, Jiang L (2020) Formulation of pH and temperature dual-responsive pickering emulsion stabilized by chitosan-based microgel for recyclable biocatalysis. Carbohydr Polym 241:116373
Shi S, Zhang L, Wang T, Wang Q, Gao Y, Wang N (2013) Poly (N-isopropylacrylamide)–Au hybrid microgels: synthesis, characterization, thermally tunable optical and catalytic properties. Soft Matter 9(46):10966–10970
Wu W, Shen J, Li Y, Zhu H, Banerjee P, Zhou S (2012) Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 33(29):7115–7125
Agrawal G, Schürings MP, Van Rijn P, Pich A (2013) Formation of catalytically active gold–polymer microgel hybrids via a controlled in situ reductive process. J Mater Chem A 1(42):13244–13251
Farooqi ZH, Iqbal S, Khan SR, Kanwal F, Begum R (2014) Cobalt and nickel nanoparticles fabricated p (NIPAM-co-MAA) microgels for catalytic applications. E-Polymers 14(5):313–321
Farooqi ZH, Khan A, Siddiq M (2011) Temperature-induced volume change and glucose sensitivity of poly [(N-isopropylacry-lamide)-co-acrylamide-co-(phenylboronic acid)] microgels. Polym Int 60(10):1481–1486
Farooqi ZH, Khan HU, Shah SM, Siddiq M (2017) Stability of poly (N-isopropylacrylamide-co-acrylic acid) polymer microgels under various conditions of temperature, pH and salt concentration. Arab J Chem 10(3):329–335
Naeem H, Farooqi ZH, Shah LA, Siddiq M (2012) Synthesis and characterization of p (NIPAM-AA-AAm) microgels for tuning of optical properties of silver nanoparticles. J Polym Res 19(9):1
Knapczyk J, Krowczynski L, Krzck J, Brzeski M, Nirnberg E, Schenk D, Struszcyk H (1989) Requirements of chitosan for pharmaceutical and biomedical applications Chitin and Chitosan Sources Chemistry, Biochemistry, Physical Properties and Applications. Elsevier, London, pp 657–663
Tozaki H, Komoike J, Tada C, Maruyama T, Terabe A, Suzuki T, Yamamoto A, Muranishi S (1997) Chitosan capsules for colonspecific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci 86:1016–1021
Kurakula M, Gorityala S, Moharir K (2020) Recent trends in design and evaluation of chitosan-based colon targeted drug delivery systems: Update 2020. J Drug Deliv Sci Technol 64:102579
Makhlof A, Tozuka Y, Takeuchi H (2009) pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm 72(1):1–8
Jain A, Jain SK (2008) In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur J Pharmaceut Sci 35:404–416
Venkatesan PN, Puvvada R, Dash BN, Prashanth Kumar D, Sarkar B, Azab A, Pathak SC, Kundu PB, Fisher MM (2011) The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 32:3794–3806
Preparation and Characterization of Metronidazole-loaded Chitosan Nanoparticles for Drug Delivery Application - Elzatahry - 2008 - Polymers for Advanced Technologies - Wiley Online Library, (n.d.). www.onlinelibrary wiley.com (Accessed November 15, 2020)
Qu Y, Chu BY, Peng JR, Liao JF, Qi TT, Shi K, Qian ZY (2015) A biodegradable thermo-responsive hybrid hydrogel: therapeutic applications in preventing the post-operative recurrence of breast cancer. NPG Asia Mate 7:e207–e207
Carreira AS, Gonçalves M, Mendonça V, Gil H, Coelho J (2010) Temperature and pH responsive polymers based on chitosan: applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr Polym 80:618–630
Marques NDN, Maia AMDS, Balaban RDC (2015) Development of dual-sensitive smart polymers by grafting chitosan with poly (N-isopropylacrylamide): an overview. Polimeros 25:237–246
Medeiros SF, Santos AM, Fessi H, Elaissari A (2011) Stimuli-responsive magnetic particles for biomedical applications. Int J Pharm 403:139–161
Cheng R, Meng F, Deng C, Klok HA, Zhong Z (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657
Patil AS, Gadad AP, Hiremath RD, Dandagi PM (2018) Exploration of the effect of chitosan and crosslinking agent concentration on the properties of dual responsive chitosan-g-poly (N-Isopropylacrylamide) co-polymeric particles. J Polym Environ 26:596–606
Jocic D, Tourrette A, Glampedaki P, Warmoeskerken MM (2009) Application of temperature and pH responsive microhydrogels for functional finishing of cotton fabric. Mater Technol 24:14–23
Salve R, Kumar P, Gajbhiye KR, Babu RJ, Gajbhiye V (2022) Stimuli-responsive strategies: Role of various molecules/moieties facilitating the design of stimuli-responsive nanocarriers. In Virendra Gajbhiye, Kavita R, Gajbhiye, Seungpyo Hong (eds) Stimuli-Responsive nanocarriers, Academic Press. p 29–60
Raghavendra VK, Syed ZI, Kusal KD, Mallanagouda SB (2019) 7 - Polysaccharide-based stimuli-sensitive graft copolymers for drug delivery. In Sabyasachi Maiti, Sougata Jana (eds) Polysaccharide carriers for drug delivery, Woodhead Publishing. p 155–177
de Lima NRB, Junior FGS, Roullin VG, Pal K (2021) Amphipathic Au-sulfur-poly (ethylene glycol)-b-poly (butylene succinate) system prepared by interfacial reaction as in-silico photosensitizer and antineoplastic carrier. J Drug Deliv Sci Technol 64:102584
Pardeshi S, Damiri F, Zehravi M, Joshi R, Kapare H, Prajapati MK et al (2022) Functional thermoresponsive hydrogel molecule to material design for biomedical applications. Polymers 14(15):3126
Asthana N, Pal K, Aljabali AA, Tambuwala MM, de Souza FG, Pandey K (2021) Polyvinyl alcohol (PVA) mixed green–clay and aloe vera based polymeric membrane optimization: peel-off mask formulation for skin care cosmeceuticals in green nanotechnology. J Mol Struct 1229:129592
Pal K, Si A, El-Sayyad GS, Elkodous MA, Kumar R, El-Batal AI et al (2021) Cutting edge development on graphene derivatives modified by liquid crystal and CdS/TiO2 hybrid matrix: optoelectronics and biotechnological aspects. Crit Rev Solid State Mater Sci 46(5):385–449
Muhammadi H, Ghorbanloo M, Mori M, Yahiro H (2022) Ionic liquid hydrogels based on poly (2-acrylamido-2-methyl-1-propanesulfonic Acid-co-1-vinylimidazole): a Green and efficient catalyst carrier for Ag nanoparticles in oxidation and adsorption of benzyl alcohol in water. Catalysis Lett. https://doi.org/10.1007/s10562-022-04104-1
Pal K, Asthana N, Aljabali AA, Bhardwaj SK, Kralj S, Penkova, et al (2021) A critical review on multifunctional smart materials ‘nanographene’emerging avenue: Nano-imaging and biosensor applications. Crit Rev Solid State Mater Sci 47(5):1–17
Duan C, Zhang D, Wang F, Zheng D, Jia L, Feng F, Zhang Q (2011) Chitosan-g-poly (N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int J Pharm 409:252–259
Kalimuthu S, Yadav AV (2009) Formulation and evaluation of carvedilol loaded Eudragit E 100 nanoparticles. Int J Pharm Tech Res 1:179–183
McWhinney SR, Goldberg RM, McLeod HL (2009) Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8:10–16
Lee CF, Wen CJ, Chiu WY (2003) Synthesis of poly (chitosan-N-isopropylacrylamide) complex particles with the method of soapless dispersion polymerization. J Polym Sci A Polym Chem 41:2053–2063
Stefanadis C, Chrysochoou C, Markou D, Petraki K, Panagiotakos DB, Fasoulakis C, Toutouzas PK (2001) Increased temperature of malignant urinary bladder tumors in vivo: the application of a new method based on a catheter technique. Clin Oncol 19:676–681
Stefanadis C, Chrysohoou C, Paraskevas E, Panagiotakos DB, Xynopoulos D, Dimitroulopoulos D, Toutouzas PK (2003) Thermal heterogeneity constitutes a marker for the detection of malignant gastric lesions in vivo. J Clin Gastroenterol 36:215–218
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, A.S., Ambhore, N.P., Suryawanshi, S.S. et al. Chitosan-Graft-Poly (N-Isopropylacrylamide)Co-Polymer as a Carrier for Targeted Delivery and Enhanced Catalytic Activity of Capecitabine. Top Catal 65, 2005–2020 (2022). https://doi.org/10.1007/s11244-022-01705-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01705-3