Abstract
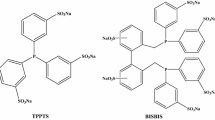
Eastman Chemical has a long term hydroformylation research program that has resulted in several successful low pressure rhodium catalyst technologies. The history of the bidentate ligand, BISBI, and the more recent development of halophosphite ligands will be discussed. The BISBI ligand is well known for high selectivity to the normal isomer and has been widely discussed in the literature. The relatively unknown halophosphite ligands exhibit a unique ability to produce aldehyde products with a variable range of linear to branched ratios by manipulating reaction variables such as reaction temperature, ligand concentration or carbon monoxide partial pressure. The halophosphite catalysts have been found to be resistant to poisoning by traditional hydroformylation poisons such as acetylene.
Similar content being viewed by others
Notes
The synthesis of the ligand and key intermediate compounds is described in a number of Eastman Chemical patents.
A sample was obtained from Aldrich Chemical Company, catalog number 370487-100G. The product has since been discontinued.
References
Slaugh L, Mullineaux R (1966) US Pat. No. 3,239,566, to Shell Oil Company
Pruett R, Smith J (1970) US Pat. 3,527,809 to Union Carbide Corporation
van Leeuwen P, Casey C, Whiteker G (2000) In: van Leeuwen P (ed) Rhodium catalyzed hydroformylation. Kluwer Academic Publishers, Boston, pp 63–96
Kamer P, Reek J, van Leeuwen P (2000) In: van Leeuwen P (ed) Rhodium catalyzed hydroformylation. Kluwer Academic Publishers, Boston, pp 35–59
Devon T, Phillips G, Puckette T, Stavinoha J, Vanderbilt J (1987) US Patents 4,694,109
Devon T, Phillips G, Puckette T, Stavinoha J, Vanderbilt J (1989) US Patents 4,851,581
Devon T, Phillips G, Puckette T, Stavinoha J, Vanderbilt J (1990) US Patents 4,904,808
Puckette T, Devon T, Phillips G, Stavinoha J (1989) US Patent 4,879,416
Puckette T (1989) US Patents 4,879,008
Puckette T (1990) US Patents 4,912,276
Puckette T (1990) US Patents 4,916,227
Puckette T (1990) US Patents 4,939,309
Puckette T (1990) US Patents 4,954,227
Puckette T (1990) US Patents 4,956,055
Puckette T (1991) US Patents 5,021,380
Puckette T (1991) US Patents 5,061,669
Klender G, Gatto V, Jones K, Calhoun C (1993) Polym Prepr 24:156
Abatjoglou A, Bryant D (1988) US Pat. 5,059,710, to Union Carbide
Oswald A, Jermasen T, Westner A, Huang I (1986) US Pat. No. 4,595,753, to Exxon Research and Engineering
Tau K (1986) US Pat. No. 4,605,781, to Celanese Corporation
Klender G (1996) Polymer durability. In: Clough R (ed) Advances in chemistry, vol 249. American Chemical Society, Washington DC, pp 396–423
Puckette T, Struck G (1998) US Patent 5,840,647, to Eastman Chemical Co., describes the bench unit and the operation of the unit in detail
Puckette T (2007) In: Schmidt S (ed) Catalysis of organic reactions. CRC Press—Taylor Francis Group, Boca Raton, pp 31–38
Liu Y, Rodgers J (2011) US Patent 7,872,156 to Eastman Chemical Company
Liu Y, Rodgers J (2011) US Patent 7,872,157 to Eastman Chemical Company
Puckette T, Liu Y (2011) US Patent 7,928,267 to Eastman Chemical Company
Puckette T (2010) US Patent Application 2010/0069679 to Eastman Chemical Company
Fell B, Beutler M (1972) Tetrahedron Lett 33:3455
Acknowledgments
Although many people have contributed to the success of this project, a few deserve special mention: Ginette Tolleson, Jody Rodgers and Y. S. Liu have been coworkers on the halophosphite work; Jerome Stavinoha, Jeff Vanderbilt, Gerry Phillips and Tom Devon as a co-workers on the BISBI work, and Eastman Chemical Company for permission to publish this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puckette, T.A. Hydroformylation Catalysis at Eastman Chemical: Generations of Catalysts. Top Catal 55, 421–425 (2012). https://doi.org/10.1007/s11244-012-9819-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-012-9819-x