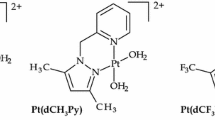
Abstract
Substitution of the coordinated water ligands from the cis-[{Pt(NH3)2H2O}2-μ-NH2(CH2) n NH2]4+ (n = 2–4, 6, 8, 10) complexes: EnPt, PropPt, ButPt, HexPt, OctPt and DecPt with S-donor nucleophiles thiourea, N,N-dimethyl-2-thiourea and N,N,N,N-tetramethyl-2-thiourea was studied under pseudo-first-order conditions as a function of concentration and temperature, using stopped-flow and UV–Vis Spectrophotometric techniques. The substitution reaction proceeded in two steps: simultaneous substitution of the aqua ligands, followed by the displacement of the ammine ligands in the trans-position due to the strong trans-effect of the coordinated thiourea nucleophiles, with each of the steps being sensitive to steric and σ-electronic properties of the alkanediamine linker. A comparison of the second-order rate constants, k 2,1st and k 2,2nd, indicates that the rate constants of the first step are 1–2 orders larger than those of the second step in all cases. The large negative ΔS ≠ values support an associative mode of substitution mechanism for both reaction steps. 1H and 195Pt NMR spectroscopy established that the α,ω-alkanediamine linkers remained coordinated to the metal centres, possibly due to their cis geometry to the incoming thiourea nucleophiles.
Graphical Abstract
The lability of aqua ligands of cis-[{Pt(NH3)2H2O}2-μ-NH2(CH2) n NH2]4+ (n = 2–10) complexes decreased from EnPt to DecPt, due to lower electrophilicity of the platinum centre caused by σ-donor effect of the (CH2) n units and to steric constraints of coordinated thiourea ligands. The experimental results are supported by density function theory (DFT) at the B3LYP/LACVP** level.















Similar content being viewed by others
Notes
Caution: Metal perchlorate complexes and perchloric acid are potentially explosive. They should be handled with care, and the complexes should be prepared in small quantities
References
Rosenberg B, Van Camp L, Trosko JE, Mansour VH (1969) Nature 222:385
Fuertes MA, Alonso C, Perez JM (2003) Chem Rev 103:645
Wang D, Lippard SJ (2005) Nat Rev Drug Discov 4:307
van Zutphen S, Reedijk J (2005) Coord Chem Rev 24:2845
Wong E, Giandomenico CM (1999) Chem Rev 99:2451
Oliver RTD (2001) Curr Opin Oncol 13:191
Jamieson ER, Lippard SJ (1999) Chem Rev 99:2467
Reedijk J (1999) Chem Rev 99:2499
Dyson PJ, Sava G (2006) Dalton Trans 1929
Abu-Surrah AS, Kettunen M (2006) Curr Med Chem 13:1337
Kelland LR (2007) Nat Rev Cancer 7:573
Reedijk J (2009) Eur J Inorg Chem 2009:1303
Soldatović T, Bugarčić ŽD, van Eldik R (2009) Dalton Trans 4526
Hall MD, Hambley TW (2002) Coord Chem Rev 232:49
Zhang CX, Lippard SJ (2003) Curr Opin Chem Biol 7:481
Ertürk H, Puchta R, van Eldik R (2009) Eur J Inorg Chem 2009:1331
Fan D, Yang X, Wang X, Zhang S, Mao J, Ding J, Lin L, Guo Z (2007) J Biol Inorg Chem 12:655 (and references therein)
Farrell N (1995) Comm Inorg Chem 16(6):373
Wheate NJ, Collins JG (2003) Coord Chem Rev 241:133
Kalayda GV, Komeda S, Ikeda K, Sato T, Chikuma M, Reedijk J (2003) Eur J Inorg Chem 24:4347
Komeda S, Kalayda GV, Lutz M, Spek AL, Sato T, Chikuma M, Reedijk J (2003) J Med Chem 46:1210
Wheate NJ, Cullinane C, Webster LK, Collins JG (2001) Anti-Cancer Drug Des 16:91
Colella G, Pennati M, Bearzatto A, Leone R, Colangelo D, Manzotti C (2001) Br J Cancer 84:1387
Jodrell DI, Evans TRJ, Steward W, Cameron D, Prendiville J, Aschele C (2004) Eur J Cancer 40:1872
Harris AL, Yang X, Hegmans A, Povirk L, Ryan JJ, Kelland L, Farrell NP (2005) Inorg Chem 44:9598
Kasparkova J, Farrell N, Brabec V (2000) J Biol Chem 275:15789
Ali MS, Whitmire KH, Toyomasi T, Siddik ZH, Khokhar AR (1999) J Inorg Biochem 77:231
Cox JW, Berners-Price SJ, Davies MS, Barlage W, Qu Y, Farrell N (2000) Inorg Chem 39:1710
Jaganyi D, Munisamy VM, Reddy D (2006) Int J Chem Kinet 38:202
Hofmann A, van Eldik R (2003) J Chem Soc Dalton Trans 2979
Mambanda A, Jaganyi D, Hochreuther S, van Eldik R (2010) Dalton Trans 39:3595
Ertürk H, Hofmann A, Puchta R, van Eldik R (2007) Dalton Trans 2295
Oehlsen ME, Qu Y, Farrell N (2003) Inorg Chem 42:5498
Montero EI, Zhang J, Moniodis JJ, Berners-Price SJ, Farrell NP (2010) Chem Eur J 16:9175
Summa N, Maigut J, Puchta R, van Eldik R (2007) Inorg Chem 46:2094
Oehlsen M, Hegmans A, Qu Y, Farrell N (2005) J Biol Inorg Chem 10:433
Williams JW, Qu Y, Bullus GH, Alvorado E, Farrell NP (2007) Inorg Chem 46:5820
Zerzankova L, Suchankova T, Vrana O, Farrell NP, Brabec V, Kasparkova J (2010) Biochem Pharmacol 79:112
Perrin DD, Armarego WLF, Perrin DR (1980) Purification of laboratory chemicals, 2nd edn. Pergamon, Oxford
Hollis LS, Amundsen AR, Stern EW (1989) J Med Chem 32:128
Qu Y, Farrell N (1992) Inorg Chem 31:930
Becke AD (1993) J Chem Phys 98:5648
Hay PJ, Wadt WR (1985) J Chem Phys 82:299
Microcal™ Origin™ (1991–2003) Version 7.5, Microcal Software, Inc., One Roundhouse Plaza, Northampton, MA, 1060, USA
Bugarčić ZD, Petrović BV, Jelić R (2001) Transit Met Chem 26:66
Appleton TG, Hall JR, Ralph SF, Thompson CMS (1984) Inorg Chem 23:3521
Zhang J, Thomas DS, Davies MS, Berners-Price SJ, Farrell N (2005) J Biol Inorg Chem 10:652
Hochreuther S, Puchta R, van Eldik R (2011) Inorg Chem 50:8984
Norman RE, Ranford J, Sadler PJ (1992) Inorg Chem 31:880 (and reference cited therein)
Kasherman Y, Sturup S, Gibson D (2009) J Biol Inorg Chem 14:387
Ma G, Min Y, Huang F, Jiang T, Liu Y (2010) Chem Commun 46:6938
Oehlsen ME, Hegmans A, Qu Y, Farrell N (2005) Inorg Chem 44:3004
Hofmann A, Jaganyi D, Munro OQ, Liehr G, van Eldik R (2003) Inorg Chem 42:1688
Mambanda A, Jaganyi D (2011) Dalton Trans 40:79
Appleton TG, Hall JR, Ralph SF, Thompson CSM (1989) Inorg Chem 28:1989
Reddy D, Jaganyi D (2011) Int J Chem Kinet 43:161
Hofmann A, Dahlengburg L, van Eldik R (2003) Inorg Chem 42:6528
Chen J-T (2006) Platinum. In: Scott RA (ed) Encyclopaedia of inorganic chemistry. Wiley, New York
Soldatović T, Jovanović S, Bugarčić ŽD, van Eldik R (2012) Dalton Trans 41:876
Ongoma OP, Jaganyi D (2013) Dalton Trans 42:2724
Ongoma OP, Jaganyi D (2013) Transit Met Chem 38:587
Ongoma OP, Jaganyi D (2013) Int J Chem Kinet 45:676
Tobe ML, Burgess J (1999) Inorganic reaction mechanisms. Addison Wesley, Longman, Ltd., Essex, pp 30–33, 70–112
Basolo F, Pearson RG (1967) Mechanisms of inorganic reactions, 2nd edn. Wiley, New York, pp 80–115
Atwood JD (1997) Inorganic and organometallic reaction mechanisms, 2nd edn. Wiley-VCH Inc., New York, pp 43–61
Acknowledgments
The authors greatly acknowledge financial support from the National Research Foundation (NRF) Pretoria, South Africa, and the University of KwaZulu-Natal. P.O.O gratefully acknowledges continued support from Egerton University, Kenya. The authors kindly thank Craig Grimmer for the support with NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11243_2014_9815_MOESM1_ESM.doc
Figures S1 to S3 show UV–Visible spectra for PropPt, ButPt and DecPt at different of pH values. Figures S4–S23, illustrate different concentration and temperature dependent studies for all nucleophiles and complexes. Table S1 shows a summary of the used wavelengths and Tables S2–S25 summarise all values of k obs determined for all reactions at different concentrations and temperatures for all nucleophiles. Included, are Figures S24 to S35 for IR, MS, 195Pt NMR spectra of the selected complexes and 13C NMR spectra for DecPt complex. (DOC 3872 kb)
Rights and permissions
About this article
Cite this article
Ongoma, P.O., Jaganyi, D. Detailed mechanistic study on ligand substitution reactions in dinuclear platinum(II) complexes: effect of alkanediamine linker. Transition Met Chem 39, 407–420 (2014). https://doi.org/10.1007/s11243-014-9815-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9815-z