Abstract
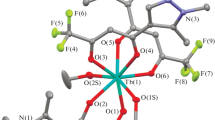
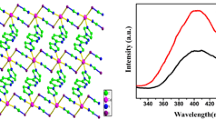
2,7-Dimethyl-1,8-naphthyridine (L1) reacts with pentacarbonylchlororhenium in toluene or chloroform to give the target complex fac-{ReCl(CO)3(L1)}. X-ray crystallographic data were obtained for fac-{ReCl(CO)3(L1)}. The structural and 1H NMR data suggest that the ligand coordinates to the rhenium in a bidentate fashion in both solid and solution states. The complex was also found to be luminescent in both solution and solid states. The fluxionality of the ligand in solution causes ligand-centred emission to be observed in solution, whereas only 3MLCT emission was observed in the solid state. Although the complex was air-stable, the lability of L1 was studied in 1H NMR experiments where CD3OD induced complete ligand dissociation over the course of 24 h, and also in reaction of fac-{ReCl(CO)3(L1)} with one equivalent of 2,2′-bipyridine in chloroform which resulted in quantitative ligand exchange.



Similar content being viewed by others
References
Cavanaugh MA, Cappo VM, Alexander CJ, Good ML (1976) Inorg Chem 15:2615. doi:10.1021/ic50165a009
Crosby GA (1975) Acc Chem Res 8:231. doi:10.1021/ar50091a003
Meyer TJ (1989) Acc Chem Res 22:163. doi:10.1021/ar00161a001
Hagfeldt A, Grätzel M (2000) Acc Chem Res 33:269. doi:10.1021/ar980112j
Gatteschi D, Mealli C, Sacconi L (1973) J Am Chem Soc 95:2736. doi:10.1021/ja00789a083
Bencini A, Berti E, Caneschi A, Gatteschi D, Giannasi E, Inverizzi I (2002) Chem Eur J 8:3660. doi:10.1002/1521-3765(20020816)8:16<3660::AID-CHEM3660>3.0.CO;2-H
Campos-Fernandez CS, Thomas LM, Galan-Mascaros JR, Xiang OY, Dunbar KR (2002) Inorg Chem 41:1523. doi:10.1021/ic010996u
Tomon T, Koizumi TA, Tanaka K (2005) Angew Chem Int Int Ed 44:229
Nakajima H, Nagano H, Tanaka K (1996) J Chem Soc Dalton Trans 1405. doi:10.1039/dt9960001405
Dewan JC, Kepert DL, White AH (1974) J Chem Soc Dalton Trans 1405
Dewan JC, Kepert DL, White AH (1974) J Chem Soc Dalton Trans 1949
Koizumi T, Tanaka K (2004) Inorg Chim Acta 357:3666. doi:10.1016/j.ica.2004.05.021
Mukkala VM, Sund C, Kwiatkowski M, Pasanen P, Hogberg M, Kankare J, Takalo H (1992) Helv Chim Acta 75:1621. doi:10.1002/hlca.19920750517
He C, Lippard SJ (2000) Tetrahedron 56:8245. doi:10.1016/S0040-4020(00)00748-1
Kuzelka J, Mukhopadhyay S, Springler B, Lippard SJ (2003) Inorg Chem 42:6447. doi:10.1021/ic0345976
Kuzelka J, Farrell JR, Lippard SJ (2003) Inorg Chem 42:8652. doi:10.1021/ic034928e
He C, Lippard SJ (2001) Inorg Chem 40:1414. doi:10.1021/ic000975k
He C, Barrios AM, Lee D, Kuzelka J, Davydov RM, Lippard SJ (2000) J Am Chem Soc 122:12683. doi:10.1021/ja0026861
Fahrni CJ, Pfatz A, Neuburger M, Zehnder M (1998) Helv Chim Acta 81:507. doi:10.1002/hlca.19980810305
Moya SA, Schmidt R, Pastene R, Sartori R, Muller U, Frenzen G (1996) Organometallics 15:3463. doi:10.1021/om960107o
Lu W, Zhang L, Ye X, Su J, Yu Z (2006) Tetrahedron 62:1806. doi:10.1016/j.tet.2005.11.048
Kukrek A, Wang D, Hou Y, Zong R, Thummel R (2006) Inorg Chem 45:10131. doi:10.1021/ic061022a
Monkowius U, Svartsov YN, Fischer T, Zabel M, Yersin H (2007) Inorg Chem Commun 10:1473. doi:10.1016/j.inoche.2007.09.010
Amoroso AJ, Coogan MP, Dunne JE, Fernández-Moreira V, Hess JB, Hayes AJ, Lloyd D, Millet C, Pope SJA, Williams C(2007) Chem Commun 3066. doi:10.1039/b706657k
Amoroso AJ, Coogan MP, Fernández-Moreira V, Hayes AJ, Lloyd D, Millet C, Pope SJA (2008) New J Chem 32:1097. doi:10.1039/b802215a
Mullice LA, Laye RH, Harding LP, Buurma NJ, Pope SJA (2008) New J Chem 32:2140. doi:10.1039/b800999f
Thorp-Greenwood FL, Coogan MP, Laye RH, Hallett AJ, Pope SJA (2009) J Organomet Chem 694:1400. doi:10.1016/j.jorganchem.2008.12.048
Coogan MP, Fernández-Moreira V, Hess JB, Pope SJA, Williams C (2009) New J Chem. doi: 10.1039/b819453j
Paudler WW, Kress TJ (1967) J Org Chem 32:833. doi:10.1021/jo01278a079
Utermohlen WP Jr (1943) J Org Chem 8:544. doi:10.1021/jo01194a009
Wrighton MS, Morse DL (1974) J Am Chem Soc 96:998. doi:10.1021/ja00811a008
SHELXL-PC Package (1998) Bruker Analytical X-ray Systems: Madison, WI
Worl LA, Duesing R, Chen P, Ciana LD, Meyer TJ (1991) J Chem Soc Dalton Trans 849. doi:10.1039/dt9910000849
Pope SJA, Coe BJ, Faulkner S (2004) Chem Commun 1551
Pope SJA, Coe BJ, Faulkner S, Laye RH (2005) Dalton Trans 1482. doi:10.1039/b500849b
Acknowledgements
We gratefully acknowledge the Universities of Cardiff and Sheffield for support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
This file is unfortunately not in the Publisher's archive anymore: (CIF 24 kb)
Rights and permissions
About this article
Cite this article
Andrews, M., Laye, R.H. & Pope, S.J.A. A luminescent rhenium(I) complex of 2,7-dimethyl-1,8-naphthyridine: synthesis, spectroscopy and X-ray crystal structure. Transition Met Chem 34, 493–497 (2009). https://doi.org/10.1007/s11243-009-9221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9221-0