Abstract
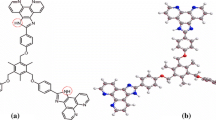
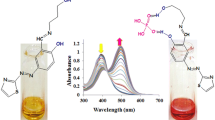
When one or more nitro groups are incorporated into an anion receptor, intramolecular proton transfer from nearby –NH groups often takes place and is a significant obstacle for the construction of supramolecular systems. In this paper, by employing an intramolecular hydrogen bond between –NH and nitro groups in order to reduce the acidity of the amide group, we were able to construct a receptor that formed stable supramolecular systems. The receptor forms a 1:2 supramolecular compound with fluoride, whereas 1:1 complexes were formed with AcO− and H2PO4 − in DMSO solution. Using this system, an easy-to-prepare test paper for the detection of F− in low concentration at (10−4 M) in water was developed.











Similar content being viewed by others
References
Bianehi A, Bowman JK (1997) In: Garcia-Espana E (ed) Supramolecular chemistry of anions. Wiley-VCH, New York
Stibor I, Zlatusková P (2005) Top Curr Chem 255:97–124
Martínez-Máñez R, Sancenón F (2003) Chem Rev 103:4419–4476. doi:10.1021/cr010421e
Ebans LS, Gale PA, Lightm ME, Quesada R (2006) Chem Commun 965
Fabbrizzi L, Licchelli M, Rabaioli G, Taglietti A (2000) Coord Chem Rev 205:85. doi:10.1016/S0010-8545(00)00239-3
Christianson DW, Lipscomb WN, Arboxypeptidase A (1989) Acc Chem Res 22:62. doi:10.1021/ar00158a003
Beer PD, Gale PA (2001) Angew Chem Int Ed 40:486. doi:10.1002/1521-3773(20010202)40:3<486::AID-ANIE486>3.0.CO;2-P
Wu FY, Wen ZC, Zhou N, Zhao YF, Jiang YB (2004) Org Lett 4:3203. doi:10.1021/ol026357k
Lee DH, Lee HY, Hong JI (2002) Tetrahedron Lett 43:7273. doi:10.1016/S0040-4039(02)01455-7
Amendola V, Boiocchi M, Fabbrizzi L, Palchetti A (2005) Chem Eur J 11:120–127. doi:10.1002/chem.200400592
Han F, Bao YH, Yang Z et al (2007) Chem Eur J 13:2880. doi:10.1002/chem.200600904
Yu XD, Lin H, Cai ZS, Lin HK (2007) Tetrahedron Lett 48:8615–8618. doi:10.1016/j.tetlet.2007.10.034
Amendola V, Boiocchi M, Fabbrizzi L, Palchetti A (2005) Chem Eur J 11:120–123. doi:10.1002/chem.200400592
Boiocchi M, Del B (2004) J Am Chem Soc 126:16507–16510. doi:10.1021/ja045936c
Saravanakumara D, Devaraja S, Iyyampillaib S (2007) Tetrahedron Lett. doi:10.1016/j.tetlet.2007.11.006
Beer PD, Szemes F, Balzani V, Salà CM et al (1997) J Am Chem Soc 119:11864. doi:10.1021/ja9725099
Valeur B, Pouget J, Bouson J, Kaschke M, Ernsting NP (1992) J Phys Chem 96:6545. doi:10.1021/j100195a008
Lin ZH, Ou SJ, Duan CY, Zhang BG, Bai ZP (2006) Chem Commun 624–626
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 20371028; 20671052).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, X., Lin, H. & Lin, H. A colorimetric anion receptor containing the cis-Ru(bipy)2 2+ group. Transition Met Chem 33, 829–834 (2008). https://doi.org/10.1007/s11243-008-9118-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-008-9118-3