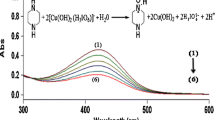
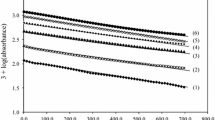
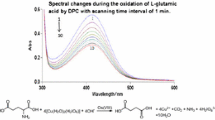
Abstract
The kinetics of oxidation of l-lysine by diperiodatoargentate(III) (DPA) in aqueous alkaline medium at a constant ionic strength of 0.50 mol dm−3 was studied spectrophotometrically. The oxidation products are aldehyde, 5-aminopentanal and Ag(I). The main products were identified by spot test, IR and GC-MS. The stoichiometry is [l-lysine]:[DPA] = 1:1. The reaction is first order with respect to diperiodatoargentate(III) concentrations, whereas the order with respect to l-lysine and alkali concentrations changes from first order to zero order as the l-lysine and alkali concentrations are increased. The effects of added products, periodate, ionic strength, and dielectric constant of the reaction medium were investigated. Based on the experimental results, a mechanism involving complex formation between DPA species and l-lysine is proposed. The reaction constants involved in the mechanism were evaluated. The activation parameters with respect to the slow step of the mechanism were determined and discussed.





Similar content being viewed by others
References
Mahadevappa DS, Rangappa KS, Gouda NM, Thimmegowda B (1982) Int J Chem Kinet 14:1183
(a) Mahanti MK, Laloo D (1990) J Chem Soc Dalton Trans 311; (b) Kulkarni RM, Bilehal DC, Nandibewoor ST (2003) Transition Met Chem 28:199
Sethuram B (2003) Some aspects of electron-transfer reactions involving organic molecules. Allied Publishers (P) Ltd., New Delhi, p 78
Jaiswal PK, Yadava KL (1970) Talanta 17:236
(a) Jayaprakash Rao P, Sethuram B, Navaneeth Rao T (1985) React Kinet Cat Letter 29:289; (b) Venkata Krishna K, Jayaprakash Rao P (1998) Indian J Chem 37A:1106 and references therein
(a) Kumar A, Kumar P, Ramamurthy P (1999) Polyhedron 18:773; (b) Kumar A, Kumar P (1999) J Phys Org Chem 12:79; (c) Kumar A, Vaishali, Ramamurthy P (2000) Int J Chem Kinet 32:286
Veeresh TM, Hiremath CV, Nandibewoor ST (2006) J Phys Org Chem 20:55
Cohen GL, Atkinson G (1964) Inorg Chem 3:1741
Jeffery GH, Bassett J, Mendham J, Denny RC (1996) Vogel’s textbook of quantitative chemical analysis, 5th edn. ELBS, Longman, Essex, UK, pp 391, 467
Hugar GH, Nandibewoor ST (1994) Transition Met Chem 19:215
Vogel AI (1989) A text book of quantitative chemical analysis, 5th edn. ELBS, Longmen Essex, UK, p 371
(a) Agrawal MC, Upadhyay SK (1983) J Sci Ind Res 42:508; (b) Hugar GH, Nandibewoor ST (1994) Transition Met Chem 19:215
(a) Crouthumel CE, Meek HV, Martin DS, Banus CV (1949) J Am Chem Soc 71:3031; (b) Crouthamel CE, Hayes AM, Martin DS (1951) J Am Chem Soc 73:82
Jaiswal PK (1972) Analyst 1:503
(a) Moelwyn-Hughes EA (1961) Physical chemistry, 2nd edn. Pergamon Press, New York; (b) Lewis ES (1973) Investigation of rates and mechanisms of reactions, 3rd edn. A Wiley Interscience publications, pp 373
Rangappa KS, Raghavendra MP, Mahadevappa DS, Channegouda D (1998) J Org Chem 63:531
(a) Martinez M, Pitarque MA, Eldik RV (1996) J Chem Soc Dalton Trans 2665; (b) Farokhi SA, Nandibewoor ST Tetrahedron 59:7595
Lewis ES (1974) Investigations of rates and mechanisms of reactions, 3rd edn. Wiley, New York, p 415
(a) Exner O (1964) Coll Czech Chem Commun 29:1094; (b) Exner O (1972) Coll Czech Chem Commun 37:1425
Leffler JE (1955) J Org Chem 20:1202
Seregar VC, Hiremath CV, Nandibewoor ST (2006) Z Phys Chem 220:615
Hiremath CV, Kulkarni SD, Nandibewoor ST (2007) Ind Eng Chem Res 45:8029
Hiremath DC, Seregar VC, Nandibewoor ST (2007) Z Phys Chem (Communicated)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munavalli, D.S., Chimatadar, S.A. & Nandibewoor, S.T. A kinetic and mechanistic study of oxidation of l-lysine by the analytical reagent diperiodatoargentate(III) in aqueous alkaline medium. Transition Met Chem 33, 535–542 (2008). https://doi.org/10.1007/s11243-008-9069-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-008-9069-8