Abstract
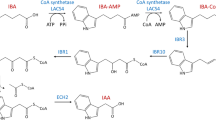
In the present study, 30 Ri-transformed root lines of peanut (Arachis hypogaea) cv. JL-24, a popular Indian cultivar, obtained following infection with Agrobacterium rhizogenes strains LBA9402, A4 and R1000 were selected on the basis of growth index and maintained in vitro for 3 years. The root lines showed high degree of branching and rapid, plagiotropic growth on phytohormone free solid N/5 medium but were devoid of root hairs. Trans-resveratrol was isolated by preparative HPLC from Ri-transformed roots and identified by ESI–MS/MS. Strain independent variability was observed among 30 Ri-transformed root lines on the basis of lateral root density per cm (7.60 ± 0.30 to 4.5 ± 0.5), relative thickness (0.54 ± 0.07 to 1.54 ± 0.1 mm), growth index (9.16 ± 1.1 to 17.79 ± 1.35 FW basis and 10.77 ± 0.95 to 19.46 ± 1.78 DW basis) and trans-resveratrol content 0.27 ± 0.03 (root line R1000-1) to 0.969 ± 0.141 mg g DW−1 (root line RIX-19) in solid N/5 medium, which was 4.1–14 fold greater than in excised non-transformed root cultures (0.06 ± 0.01 mg g DW−1). Optimum growth and productivity in liquid culture was achieved in N/5 medium supplemented with 0.01 % activated charcoal. Root line RIX-19 showed maximum trans-resveratrol accumulation (1.21 ± 0.09 mg g DW−1) and productivity (0.37 ± 0.08 mg per flask), which was 19 fold higher than non-transformed root cultures. This optimized protocol can be utilized for large scale cultivation of transformed root cultures in industrial bioreactors for mass synthesis of trans-resveratrol.










Similar content being viewed by others
References
Akasaka Y, Mii M, Daimon H (1998) Morphological alterations and root nodule formation in Agrobacterium rhizogenes-mediated transgenic hairy roots of peanut (Arachis hypogaea L.). Ann Bot 81:355–362
Almagro L, Belchı´-Navarro S, Sabater-Jara A, Vera-Urbina JC, Selle´s-Marchart S, Bru R, Pedren˜o MA (2013) Bioproduction of trans-resveratrol from grapevine cell cultures. In: Ramawat KG, Merillon JM (eds) Handbook of natural products. Springer, Berlin, pp 1683–1713
Alpizar E, Dechamp E, Lapeyre-Montes F, Guilhaumon C, Bertrand B, Jourdan C, Lashermes P, Etienne H (2008) Agrobacterium rhizogenes-transformed roots of Coffee (Coffea arabica): conditions for long-term proliferation, and morphological and molecular characterization. Ann Bot 101:929–940
Aoki T, Matsumoto H, Asako Y, Matsunaga Y, Shimomura K (1997) Variation of alkaloid productivity among several clones of hairy roots and regenerated plants of Atropa belladonna transformed with Agrobacterium rhizogenes 15834. Plant Cell Rep 16:282–286
Batra J, Dutta A, Singh D, Kumar S, Sen J (2004) Growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root clones in relation to left- and right-termini-linked Ri T-DNA gene integration. Plant Cell Rep 23:148–154
Baur JA, Pearson KJ, Price NL et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nat 444:337–342
Bishi SK, Lokesh K, Mahatma MK, Khatediya N, Chauhan SM, Misra JB (2015) Quality traits of Indian peanut cultivars and their utility as nutritional and functional food. Food Chem 167:107–114
Bonhomme V, Laurain-Mattar D, Lacoux J, Fliniaux M, Dubreuil AJ (2000) Tropane alkaloid production by hairy roots of Atropa belladonna obtained after transformation with Agrobacterium rhizogenes 15834 and Agrobacterium tumefaciens containing rol A, B, C genes only. J Biotechnol 81:151–158
Camont L, Cottart CH, Rhayem Y, Nivet-Antoine V, Djelidi R, Collin F, Beaudeux JL, Bonnefont-Rousselot D (2009) Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal Chim Acta 634:121–128
Cardarelli M, Spano L, Mariotti D, Mauro ML, Van Sluys MA, Constantino P (1985) Identification of the genetic locus responsible for non-polar root induction by Agrobacterium rhizogenes. Plant Mol Biol 5:385–391
Chandra S (2012) Natural plant genetic engineer Agrobacterium rhizogenes: role of T-DNA in plant secondary metabolism. Biotechnol Lett 34:407–415
Chandran RP, Potty VP (2008) Induction of hairy roots through the mediation of four strains of Agrobacterium rhizogenes on five host plants. Indian J Biotechnol 7:122–128
Chaudhuri KN, Ghosh B, Tepfer D, Jha S (2005) Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: growth and tylophorine productivity in different transformed root clones. Plant Cell Rep 24:25–35
Chen RS, Wu PL, Chiou RYY (2002) Peanut roots as a source of resveratrol. J Agric Food Chem 50:1665–1667
Chen Y, Tseng SH, Lai HS, Chen WJ (2004) Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 136:57–66
Chen X, He H, Wang G, Yang B, Ren W, Ma L, Yu Q (2007) Stereospecific determination of cis- and trans-resveratrol in rat plasma by HPLC: application to pharmacokinetic studies. Biomed Chromatogr 21:257–265
Christensen B, Sriskandarajah S, Serek M, Müller R (2008) Transformation of Kalanchoe blossfeldiana with rol-genes is useful in molecular breeding towards compact growth. Plant Cell Rep 27:1485–1495
Condori J, Sivakumar G, Hubstenberger J, Dolan MC, Sobolev VS, Medina-Bolivar F (2010) Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: effects of culture medium and growth stage. Plant Physiol Biochem 48:310–318
Delmas D, Lançon A, Colin D, Jannin B, Latruffe N (2006) Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets 7:423–442
Deus B, Zenk MH (1982) Exploitation of plant cells for the production of natural compounds. Biotechnol Bioeng 24:1965–1974
Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B (2007) Effects of resveratrol in inflammatory arthritis. Inflammation 30:1–2
Frémont L (2000) Biological effects of resveratrol. Life Sci 66:663–673
Geng L, Niu L, Gresshoff PM, Shu C, Song F, Huang D, Zhang J (2012) Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants in peanut (Arachis hypogaea L.). Plant Cell Tiss Organ Cult 109:491–500
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185
Guillon S, Jocelyne TG, Pati PK, Marc R, Pascal G (2006) Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol 24:403–409
Gupta R, Pandey P, Singh S, Singh DK, Saxena A, Luqman S, Bawankule DU, Banerjee S (2015) Advances in Boerhaavia diffusa hairy root technology: a valuable pursuit for identifying strain sensitivity and up-scaling factors to refine metabolite yield and bioactivity potentials. Protoplasma. doi:10.1007/s00709-015-0875-5
Holden PR, Holden MA, Yeoman MM (1988) Variation in the secondary metabolism of cultured plant cells. In: Robins RJ, Rhodes MJC (eds) Manipulating secondary metabolism in culture. Cambridge University Press, Cambridge, pp 15–29
Hooykaas PJ, Klapwjik PM, Nuit MP, Schilperoot RA, Hirsch A (1977) Transfer of the A. tumifaciens Ti plasmid to avirulent Agrobacteria and Rhizobium ex planta. J Gen Microbiol 98:477–484
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nat 425:191–196
Hsieh TC, Wu JM (2010) Resveratrol: biological and pharmaceutical properties as anticancer molecule. BioFactors 36:360–369
Jeandet P, Cl´ement C, Courot E (2014) Resveratrol production at large scale using plant cell suspensions. Eng Life Sci 14:622–632
Jha S, Bandyopadhyay M, Chaudhuri KN, Ghosh S, Ghosh B (2005) Biotechnological approaches for the production of forskolin, withanolides, colchicine and tylophorine. Plant Genet Resour Charact Util 3:101–115
Jouanin L, Tourneur J, Tourneur C, Casse-Delbart F (1986) Restriction maps and homologies of the three plasmids of Agrobacterium rhizogenes strain A4. Plasmid 16:124–134
Jouanin L, Guerche D, Pamboukdjian N, Tourneur C, Casse-Delbart F, Tourneur J (1987) Structure of T-DNA in plants regenerated from roots transformed by Agrobacterium rhizogenes strain A4. Mol Gen Genet 206:387–392
Jung G, Tepfer D (1987) Use of genetic transformation by the Ri T-DNA of Agrobacterium rhizogenes to stimulate biomass and tropane alkaloid production in Atropa belladonna and Calystegia sepium roots. Plant Sci 50:145–151
King RE, Bomser JA, Min DB (2006) Bioactivity of resveratrol. Compr Rev Food Sci F 5:65–70
Lan X, Quan H (2010) Hairy root culture of Przewalskia tangutica for enhanced production of pharmaceutical tropane alkaloids. J Med Plants Res 4:1477–1481
Larronde F, Richard T, Delaunay JC, Decendit A, Monti JP, Krisa S, Mérillon JM (2005) New stilbenoid glucosides isolated from Vitis vinifera cell suspension cultures (cv. Cabernet Sauvignon). Planta Med 71:888–890
Liu C, Wang L, Wang J, Wu B, Liu W, Fan P, Liang Z, Li S (2013) Resveratrols in Vitis berry skins and leaves: their extraction and analysis by HPLC. Food Chem 136:643–649
Lyons MM, Yu C, Toma RB, Cho SY, Reiboldt W, Lee J, van Breemen RB (2003) Resveratrol in raw and baked blueberries and bilberries. J Agric Food Chem 51:5867–5870
Madhusudhanan K, Rahiman BA (2000) The effect of activated charcoal supplemented media to browning of in vitro cultures of Piper species. Biol Plant 43:297–299
Majumdar S, Garai S, Jha S (2011) Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopasaponins in transformed calli and plants. Plant Cell Rep 30:941–954
Malovaná S, Montelongo FJG, Pérez JP, Rodríguez-Delgado MA (2001) Optimisation of sample preparation for the determination of trans-resveratrol and other polyphenolic compounds in wines by high performance liquid chromatography. Anal Chim Acta 428:245–253
Medina-Bolivar F, Condori J, Rimando AM, Hubstenberger J, Shelton K, O’Keefe SF, Bennett S, Dolan MC (2007) Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 68:1992–2003
Montsko G, Nikfardjam MSP, Szabo Z, Boddi K, Lorand T, Ohmacht R, Mark L (2008) Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC-APCI-MS. J Photochem Photobiol A Chem 196:44–50
Moore L, Warren G, Strobel G (1979) Involvement of a plasmid in the hairy root disease of plants caused by Agrobacterium rhizogenes. Plasmid 2:617–626
Moyano E, Fornalé S, Palazón J, Cusidó RM, Bonfill M, Morales C, Piñol MT (1999) Effect of Agrobacterium rhizogenes T-DNA on alkaloid production in Solanaceae plants. Phytochemistry 52:1287–1292
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180:439–446
Pan MJ, Staden JV (1998) The use of charcoal in in vitro culture—a review. Plant Growth Regul 26:155–163
Petit A, David C, Dahl GA, Ellis JG, Guyon P, Casse-Delbart F, Tempé J (1983) Further extension of the opine concept: plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet 190:204–214
Ray S, Majumder A, Bandyopadhyay M, Jha S (2014) Genetic transformation of sarpagandha (Rauvolfia serpentina) with Agrobacterium rhizogenes for identification of high alkaloid yielding lines. Acta Physiol Plant 36:1599–1605
Rodríguez-Delgado MA, González G, Pérez-Trujillo JP, García-Montelongo FJ (2002) Trans-resveratrol in wines from the Canary Islands (Spain). Analysis by high performance liquid chromatography. Food Chem 76:371–375
Roychowdhury D, Majumder A, Jha S (2013) Agrobacterium rhizogenes-mediated transformation in medicinal plants: prospects and challenges. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants. Springer, Berlin, pp 29–66
Roychowdhury D, Basu A, Jha S (2015) Morphological and molecular variation in Ri-transformed root lines are stable in long term cultures of Tylophora indica. Plant Growth Regul 75:443–453
Sales JM, Resurreccion AVA (2014) Resveratrol in peanuts. Crit Rev Food Sci Nutr 54:734–770
Sanders TH, McMichael RW Jr, Hendrix KW (2000) Occurrence of resveratrol in edible peanuts. J Agric Food Chem 48:1243–1246
Setamam NM, Sidik NJ, Rahman ZA, Zain CRCM (2014) Induction of hairy roots by various strains of Agrobacterium rhizogenes in different types of Capsicum species explants. BMC Res Notes 7:414–422
Sevón N, Dräger B, Hiltunen R, Oksman-Caldentey KM (1997) Characterization of transgenic plants derived from hairy roots of Hyoscyamus muticus. Plant Cell Rep 16:605–611
Sinharoy S, Saha S, Chaudhury SR, DasGupta M (2009) Transformed hairy roots of Arachis hypogea: a tool for studying root nodule symbiosis in a non-infection thread legume of the Aeschynomeneae tribe. Mol Plant Microbe Interact 22:132–142
Sivakumar G, Yu KW, Paek KY (2005) Production of biomass and ginsenoides from adventitious roots of Panax ginseng in bioreactor cultures. Eng Life Sci 5:333–342
Sivakumar G, Liu C, Towler MJ, Weathers PJ (2010) Biomass production of hairy roots of Artemisia annua and Arachis hypogaea in a scaled-up mist bioreactor. Biotechnol Bioeng 107:802–813
Sokal RR, Rohlf FJ (1987) Introduction to biostatistics. WH Freeman, New York
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41:375–383
Taneja J, Jaggi M, Wankhede DP, Sinha AK (2010) Effect of loss of T-DNA genes on MIA biosynthetic pathway gene regulation and alkaloid accumulation in Catharanthus roseus hairy roots. Plant Cell Rep 29:1119–1129
Tepfer D (1984) Genetic transformation of several species of higher plants by Agrobacterium rhizogenes: phenotypic consequences and sexual transmission of the transformed genotype and phenotype. Cell 37:959–967
Tepfer D (1995) Agrobacterium rhizogenes-mediated transformation: transformed roots to transformed plants. In: Potrykus I, Spangenberg G (eds) Gene transfer to plants. Springer, Berlin, pp 45–52
Tian L (2015) Using hairy roots for production of valuable plant secondary metabolites. In: Advances in biochemical engineering/biotechnology. Springer, Berlin. doi:10.1007/10_2014_298
Trela BC, Waterhouse AL (1996) Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem 44:1253–1257
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for production of plant secondary metabolites. Phytochem Rev 1:13–25
Vervliet G, Holsters M, Teuchy H, Van Montagut M, Schell J (1975) Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. J Gen Virol 26:33–48
Wang YM, Wang JB, Luo D, Jia JF (2001) Regeneration of plants from callus cultures of roots induced by Agrobacterium rhizogenes on Alhagi pseudalhagi. Cell Res 11:279–284
Weatherhead MA, Burdon J, Henshaw GG (1979) Effects of activated charcoal as an additive plant tissue culture media: part 2. Z Pflanzenphysiol 94:399–405
Weathers PJ, Towler MJ, Xu J (2010) Bench to batch: advances in plant cell culture for producing useful products. Appl Microbiol Biotechnol 85:1339–1351
White FF, Nester EW (1980) Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol 41:1134–1141
Xu Q, Si LY (2012) Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action. Nutr Res 32:648–658
Xu J, Ge X, Dolan MC (2011) Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol Adv 29:278–299
Yang T, Fang L, Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Balmaceda C, Medina-Bolivar F (2015) Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J Agric Food Chem 63:3942–3950
Acknowledgments
MH is grateful to the University Grants Commission, New Delhi, for the award of Junior (Sanction No.—UGC/1015/Jr. Fellow (Sc.); date-15.10.09) and Senior Research Fellowship (sanction No.—UGC/430/Jr. Fellow; date-16.04.12) and expresses his sincere gratitude to Prof M. Dasgupta, Department of Biochemistry, C.U., for her guidance and encouragement. Thanks are due to Mr. Sudip Saha for his help in HPLC analysis. We also thank the Coordinator, DBT-IPLS, University of Calcutta for providing ESI–MS/MS facilities.
Author contribution
MH and SJ conceived and designed research.MH did all the experiments and analyzed the data. SJ and MH wrote the final manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Expression of Ri T-DNA genes at the transcription level in Ri-transformed root lines by RT- PCR analysis using rolA, rolB, rolC and rolD primers. Lane 1: molecular marker (1000 bp plus DNA ladder), lane 2: positive control (pLJ1), lane 3: negative control (genomic DNA of NTH) lanes 4-17: genomic DNA of Ri-transformed root lines (TIFF 1493 kb)
Fig. S2
Variation in growth of 30 Ri-transformed root lines after 28 days on solid N/5 medium (a) Growth index based on fresh weight; (b) Growth index based on dry weight. Bars with the same letters are not significantly different at p ≤ 0.05 according to ANOVA and DMRT for each data point (TIFF 1454 kb)
Fig. S3
Variation in primary root elongation of 30 Ri-transformed root lines after 28 days. Bars with the same letters are not significantly different at p ≤ 0.05 according to ANOVA and DMRT for each data point (TIFF 1994 kb)
Fig. S4
Growth kinetics of two randomly selected Ri-transformed root lines RIX-23 and RIX-33 cultured on solid N/5 medium showing root tip elongation (mm) per day. All values are the means ± standard deviation of three independent experiments (n = 15) (TIFF 566 kb)
Rights and permissions
About this article
Cite this article
Halder, M., Jha, S. Enhanced trans-resveratrol production in genetically transformed root cultures of Peanut (Arachis hypogaea L.). Plant Cell Tiss Organ Cult 124, 555–572 (2016). https://doi.org/10.1007/s11240-015-0914-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0914-0