Abstract
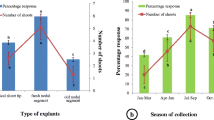
Leaf explants of the second or third node were collected from field-grown elite Jatropha curcas trees and incubated in Murashige and Skoog’s (Physiol Plant 15:473–497, 1962) medium supplemented with growth regulators. Direct shoot organogenesis was induced when explants were incubated in a medium containing 0.5 mg l−1 benzyladenine (BA) and 0.1 mg l−1 indolebutyric acid (IBA). A maximum of seven shoot buds differentiated within 6 weeks of culture incubation. Indirect shoot organogenesis was obtained when explants were incubated in the medium supplemented with 0.5 mg l−1 BA along with 1.0 mg l−1 each of 2,4-dichlorophenoxyacetic acid (2,4-D) and indoleacetic acid (IAA). A pulse treatment of 0.5 mg l−1 thidiazurone (TDZ) and 0.1 mg l−1 IBA for 5 days was necessary for shoot organogenesis in green compact callus before subculture into 0.5 mg l−1 BA and 0.1 mg l−1 IBA containing medium. Leaf explants of J. curcas, collected from the field, contained endophytic bacterial contamination, which expressed itself after 2–3 subcultures. These bacteria were cultured and identified as Enterobacter ludwigii. After staining, these were found as gram-negative bacteria. Their sensitivity against different antibiotics has been tested by culturing them with different antibiotic stabs for 72 h. Finally, Augmentin® was found as the most effective and suitable antibiotic which not only controlled the bacteria within 2–3 subcultures but also supported the regeneration system and growth of the regenerated shoots and such cultures have been grown for a long-term of over 2 years without any contamination.





Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- Kn:
-
Kinetin
- MS:
-
Murashige and Skoog’s (1962) medium
- NAA:
-
α-Naphthaleneacetic acid
- TDZ:
-
Thidiazurone
References
Brisibe EA, Gajdosova A, Olesen A, Andersen SB (2000) Cytodifferentiation and transformation of embryogenic callus lines derived from anther culture of wheat. J Expl Bot 51:187–196
Bush DR, Leach JE (2007) Translational genomics for bioenergy production: there’s room for more than one model. Plant Cell 19:2971–2973
Chhetri AB, Tango MS, Budge SM, Watts KC, Islam MR (2008) Non-edible plant oils as new sources for biodiesel production. Int J Mol Sci 9:169–180
Datta MM, Mukherjee P, Ghosh B, Jha TB (2007) In vitro clonal propagation of biodiesel plant (Jatropha curcas L.). Curr Sci 93:1438–1442
Fairless D (2007) Biofuel: the little shrub that could—may be. Nature 449:652–655
Forson FK, Oduro ED, Hammond-Donkoh E (2004) Performance of Jatropha oil blends in a diesel engine. Renew Energ 29:1135–1145
Gressel J (2008) Transgenics are imperative for biofuel crops. Plant Sci 174:246–263
Ieamkhang S, Chatchawankanphanich O (2005) Augmentin® as an alternative antibiotic for growth suppression of Agrobacterium for tomato (Lycopersicon esculentum) transformation. Plant Cell Tiss Org Cult 82:213–220
Jha TB, Mukherjee P, Datta MM (2007) Somatic embryogenesis in Jatropha curcas linn., an important biofuel plant. Plant Biotechnol Rep 1:135–140
Keith O (2000) A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass & Bioenerg 19:1–15
Kulkarni AA, Kelkar SM, Watve MG, Krishnamurthy KV (2007) Characterization and control of endophytic bacterial contaminants in in vitro cultures of Piper spp., Taxus baccata subsp. wallichiana, and Withania somnifera. Can J Microbiol 53:63–74
Li K, Yang W-Y, Li L, Zhang CH, Cui Y-Z, Sun Y-Y (2007) Distribution and development strategy for Jatropha curcas L. For Stud China 9:120–126
Lichtenstein C, Draper J (1986) Genetic engineering of plants. In: Glover DM (ed) DNA cloning: a practical approach. IRL Press, Oxford/Washington, pp 67–119
Lin J, Tang L, Chen F (2002) Tissue culture and plantlet regeneration of Jatropha curcas. Plant Physiol Comm 38:252
Lu WD, Wei Q, Tang L, Yan F, Chen F (2003) Induction of callus from Jatropha curcas and rapid propagation. Chin J Appl Environ Biol 9:127–130
Misra P, Kochhar S (2008) Acclimatization of in vitro-raised asiatic hybrid lily plants in subtropical climate and associate changes in stress proteins and antioxidant enzymes. J Plant Biochem Biotechnol 17:45–50
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pirttilä AM, Podolich O, Koskimäki JJ, Hohtola E, Hohtola A (2008) Role of origin and endophyte infection in browning of bud-derived tissue cultures of scots pine (Pinus sylvestris L.). Plant Cell Tiss Organ Cult 95:47–55
Rajore S, Batra A (2005) Efficient plant regeneration via shoot tip explant in Jatropha curcas L. J Plant Biochem Biotechnol 14:73–75
Reed BM, Buckley PM, DeWilde TN (1995) Detection and eradication of endophytic bacteria from micropropagated mint plants. In Vitro Cell Dev Biol-Plant 31:53–57
Runge CF, Senauer B (2007) Biofuel: corn isn’t the king of this growing domain. Nature 450:478
Santana JRF de, Paiva R, Aloufa MAI, Lemos EEP (2003) Efficiency of ampicillin and benomyl at controlling contamination of annonaceae leaf segments cultured in vitro. Fruits: Paris 58:357–361
Sujatha M, Mukta N (1996) Morphogenesis and plant regeneration from tissue cultures of Jatropha curcas. Plant Cell Tiss Org Cult 44:135–141
Sujatha M, Makkar HPS, Becker K (2005) Shoot bud proliferation from axillary nodes and leaf sections of non-toxic Jatropha curcas L. Plant Gr Reg 47:83–90
Tao H, Shaolin P, Gaofeng D, Lanying Z, Gengguang L (2002) Plant regeneration from leaf-derived callus in Citrus grandis (pummelo): effects of auxins in callus induction medium. Plant Cell Tiss Org Cult 69:141–146
Thomas P (2004) In vitro decline in plant cultures: detection of a legion of covert bacteria as the cause for degeneration of long-term micropropagated triploid watermelon cultures. Plant Cell Tiss Org Cult 77:173–179
Thomas P (2006) Isolation of an ethanol-tolerant endospore forming gram-negative Brevibacillus sp. as a covert contaminant in grape tissue cultures. J Appl Micro 101:764–774
Thomas P, Prabhakara BS, Pitchaimuthu M (2006) Cleansing the long-term micropropagated triploid watermelon cultures from covert bacteria and field testing the plants for clonal fidelity and fertility during the 7–10 year period in vitro. Plant Cell Tiss Org Cult 85:317–329
Thomas P, Kumari S, Swarna GK, Prakash DP, Dinesh MR (2007) Ubiquitous presence of fastidious endophytic bacteria in field shoots and index-negative apparently clean shoot-tip cultures of papaya. Plant Cell Rep 26:1491–1499
Thomas P, Swarna GK, Patil P (2008) Ubiquitous presence of normally non-cultivable endophytic bacteria in field shoot tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tiss Org Cult 93:39–54
Wei Q, Lu W-D, Liao Y, Pan S-L, Xu Y, Tang L, Chen F (2004) Plant regeneration from epicotyl explant of Jatropha curcas. J Plant Physiol Mol Biol 30:475–478
Acknowledgments
The authors are thankful to the Director, National Botanical Research Institute, Lucknow, for providing necessary infrastructure facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Misra, P., Gupta, N., Toppo, D.D. et al. Establishment of long-term proliferating shoot cultures of elite Jatropha curcas L. by controlling endophytic bacterial contamination. Plant Cell Tiss Organ Cult 100, 189–197 (2010). https://doi.org/10.1007/s11240-009-9636-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9636-5