Abstract
Background
Use of direct oral anticoagulants (DOACs) in patients with cancer remains suboptimal due to the concern regarding potential drug-drug interactions (DDIs) with antineoplastic treatments. However, the clinical relevance of these DDIs is unknown.
Methods
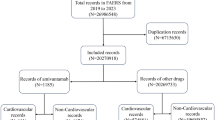
We conducted a pharmacovigilance study of adverse event (AE) reports from the US Food and Drug Administration Adverse Event Reporting System from 1/1/2004 to 12/31/2021. AE reports containing DOACs and antineoplastic agents with CYP3A4/P-gp inhibitory or inducing activity suggested by published pharmacokinetic studies were included (n = 36,066). The outcomes of interest were bleeding or stroke, identified by MedDRA dictionary version 25.0. We used disproportionality analyses (DPA), logistic regression models (LR), and Multi-item Gamma-Poisson Shrinker (MGPS) (Empirical Bayes Geometric Means (EBGM) and 90% credible intervals (90% CIs)) algorithms to identify the safety signal of DDIs.
Results
The highest bleeding reporting rates for each drug class were the combination of DOACs with neratinib (39.08%, n = 34), tamoxifen (21.22%, n = 104), irinotecan (20.54%, n = 83), and cyclosporine (19.17%, n = 227). The highest rate of stroke was found for prednisolone (2.43%, n = 113). In the primary analysis, no signal of DDIs by the antineoplastic therapeutic class was detected by MGPS, DPA, and LR approaches. By individual antineoplastic drug, DOACs-neratinib was the only signal detected [EBGM (EB05-EB95) = 2.71 (2.03–3.54)].
Conclusion
No signal of DDIs between DOACs and antineoplastic agents was detected, except for DOAC-neratinib. Most DDIs between DOACs and antineoplastic agents may not be clinically relevant. The DDIs between DOACs and neratinib should be further examined in future research.
Highlights
Using a pharmacovigilance approach, this study examined the adverse event reporting risk in bleeding/stroke with concurrent DOACs and antineoplastic agents (kinase inhibitors, hormonal therapy, chemotherapeutic agents, immunomodulating agents, and antineoplastic CYP3A4/P-gp inducers) from real-world data.
Most of the assessed drug-drug interactions (DDIs) between DOACs and antineoplastic agents (kinase inhibitors, hormonal therapy, chemotherapeutic agents, immunomodulating agents, and antineoplastic CYP3A4/P-gp inducers) did not exhibit significant risk signals.
The combination of DOACs and neratinib showed an elevated risk signal and should be further examined in future research.

Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are publicly available in the US FDA FAERS repository, https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
References
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122(10):1712–1723
Prandoni P, Lensing AWA, Piccioli A et al (2002) Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100(10):3484–3488
Atterman A, Friberg L, Asplund K, Engdahl J (2020) Net benefit of oral anticoagulants in patients with atrial fibrillation and active cancer: a nationwide cohort study. EP Europace 22(1):58–65
Fradley MG, Ellenberg K, Alomar M et al (2020) Patterns of Anticoagulation Use in patients with Cancer with Atrial Fibrillation and/or atrial flutter. JACC: CardioOncology 2(5):747–754
O’Neal WT, Claxton JS, Sandesara PB et al (2018) Provider Specialty, Anticoagulation, and stroke risk in patients with Atrial Fibrillation and Cancer. J Am Coll Cardiol 72(16):1913–1922
Hindricks G, Potpara T, Dagres N et al (2020) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42(5):373–498
January CT, Wann LS, Calkins H et al (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the heart rhythm society in collaboration with the Society of thoracic surgeons. Circulation 140(2):e125–e151
Delluc A, Wang T-F, Yap E-S et al (2019) Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: Guidance from the SSC of the ISTH. J Thromb Haemost 17(8):1247–1252
Menichelli D, Vicario T, Ameri P et al (2021) Cancer and atrial fibrillation: Epidemiology, mechanisms, and anticoagulation treatment. Prog Cardiovasc Dis 66:28–36
Gatti M, Raschi E, Poluzzi E et al (2020) The Complex Management of Atrial Fibrillation and Cancer in the COVID-19 era: drug interactions, thromboembolic risk, and Proarrhythmia. Curr Heart Fail Rep 17(6):365–383
Walker AJ, Card TR, West J, Crooks C, Grainge MJ (2013) Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer 49(6):1404–1413
Cronin-Fenton DP, Søndergaard F, Pedersen LA et al (2010) Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. Br J Cancer 103(7):947–953
Girardi L, Wang T-F, Ageno W, Carrier M (2023) Updates in the incidence, pathogenesis, and management of Cancer and venous thromboembolism. Arterioscler Thromb Vasc Biol 43(6):824–831
Riess H, Prandoni P, Harder S, Kreher S, Bauersachs R (2018) Direct oral anticoagulants for the treatment of venous thromboembolism in cancer patients: potential for drug–drug interactions. Crit Rev Oncol/Hematol 132:169–179
Correction to (2022) Complexity and clinical significance of drug–drug interactions (DDIs) in oncology: challenging issues in the care of patients regarding cancer–associated thrombosis (CAT). Support Care Cancer 30(10):8575–8575
Comerota AJ, Ramacciotti E (2016) A comprehensive overview of direct oral anticoagulants for the management of venous thromboembolism. Am J Med Sci 352(1):92–106
Steffel J, Verhamme P, Potpara TS et al (2018) The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39(16):1330–1393
Wang CL, Wu VC, Tu HT et al (2021) Risk of major bleeding associated with concomitant use of anticancer drugs and direct oral anticoagulant in patients with cancer and atrial fibrillation. J Thromb Thrombolysis
Keramida K, Filippatos G, Farmakis D (2021) Cancer treatment and atrial fibrillation: use of pharmacovigilance databases to detect cardiotoxicity. Eur Heart J - Cardiovasc Pharmacotherapy 7(4):321–323
U.S. Food and Drug Administration. Questions and Answers on FDA’s Adverse Event Reporting System (FAERS). https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. (2018) Accessed December 20, 2021
Food US, Administration D (2022) FDA Adverse Event Reporting System (FAERS) Public Dashboard. https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis. Published 2021. Accessed March 23,
Food US, Administration D (2021) FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Accessed December 20, 2021
The University of Liverpool (2022) Liverpool cancer drug interaction checker. https://cancer-druginteractions.org/checker. Accessed December 13, 2022
US Food and Drug Administration (2021) Purple Book: Database of Licensed Biological Products. https://purplebooksearch.fda.gov/. Accessed December 21, 2021
IBM Micromedex (2021) IBM® Micromedex® RED BOOK. https://www.micromedexsolutions.com/micromedex2/librarian/CS/64D0A6/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/FB80F0/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.FindRedBook?navitem=topRedBook&isToolPage=true. Accessed December 21, 2021
US Food and Drug Administration. National Drug Code Directory (2021) https://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory. Published 2021. Accessed December 21,
IBM Micromedex (2021) micromedexsolutions.com/micromedex2/librarian. Accessed December 21, 2021
Sakaeda T, Tamon A, Kadoyama K, Okuno Y (2013) Data Mining of the Public Version of the FDA adverse event reporting system. Int J Med Sci 10(7):796–803
MedDRA MSSO (2021) Introductory Guide for Standardised MedDRA Queries (SMQs) Version 24.1. https://alt.meddra.org/files_acrobat/SMQ_intguide_24_1_English.pdf. Accessed December 21, 2021
Ibrahim H, Saad A, Abdo A, Sharaf Eldin A (2016) Mining association patterns of drug-interactions using post marketing FDA’s spontaneous reporting data. J Biomed Inform 60:294–308
van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11(1):3–10
Noguchi Y, Tachi T, Teramachi H (2019) Review of statistical methodologies for detecting drug–drug interactions using spontaneous Reporting Systems. Front Pharmacol 10:1319
Ibrahim H, Abdo A, El Kerdawy AM, Eldin AS (2021) Signal Detection in Pharmacovigilance: a review of Informatics-driven approaches for the Discovery of Drug-Drug Interaction signals in different data sources. Artif Intell Life Sci 1:100005
US Food and Drug Administration (2021) Data Mining at FDA -- White Paper. https://www.fda.gov/science-research/data-mining/data-mining-fda-white-paper. Published 2018. Accessed December 22,
Dumouchel W (1999) Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat 53(3):177–190
Sunaga T, Cicali B, Schmidt S, Brown J (2021) Comparison of contraceptive failures associated with CYP3A4-inducing drug-drug interactions by route of hormonal contraceptive in an adverse event reporting system. Contraception 103(4):222–224
Szarfman A, Machado SG, O’Neill RT (2002) Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 25(6):381–392
Pham M, Cheng F, Ramachandran K (2019) A comparison study of Algorithms to detect drug-adverse event Associations: Frequentist, bayesian, and machine-learning approaches. Drug Saf 42(6):743–750
McAdams MA, Governale LA, Swartz L, Hammad TA, Dal Pan GJ (2008) Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf 17(9):882–889
Wheelock KM, Ross JS, Murugiah K, Lin Z, Krumholz HM, Khera R (2021) Clinician Trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open 4(12):e2137288–e2137288
Duvalyan A, Pandey A, Vaduganathan M et al (2021) Trends in Anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Association 10(24):e022644
Joy M, Williams J, Emanuel S et al (2022) Trends in direct oral anticoagulant (DOAC) prescribing in English primary care (2014–2019). Heart :heartjnl–2022
Perreault S, de Denus S, White-Guay B et al (2020) Oral anticoagulant prescription Trends, Profile Use, and determinants of adherence in patients with Atrial Fibrillation. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 40(1):40–54
Ortel TL, Neumann I, Ageno W et al (2020) American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 4(19):4693–4738
Ardeshirrouhanifard S, An H, Goyal RK et al (2022) Use of oral anticoagulants among individuals with cancer and atrial fibrillation in the United States, 2010–2016. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 42(5):375–386
Costa OS, Kohn CG, Kuderer NM, Lyman GH, Bunz TJ, Coleman CI (2020) Effectiveness and safety of rivaroxaban compared with low-molecular-weight heparin in cancer-associated thromboembolism. Blood Adv 4(17):4045–4051
Agnelli G, Becattini C, Meyer G et al (2020) Apixaban for the treatment of venous Thromboembolism Associated with Cancer. N Engl J Med 382(17):1599–1607
Raskob GE, van Es N, Verhamme P et al (2017) Edoxaban for the treatment of Cancer-Associated venous thromboembolism. N Engl J Med 378(7):615–624
Julia S, James U (2017) Direct oral anticoagulants: a Quick Guide. Eur Cardiol 12(1):40–45
Otten LS, Piet B, van den Heuvel MM et al (2022) Practical recommendations to combine small-molecule inhibitors and direct oral anticoagulants in patients with nonsmall cell lung cancer. Eur Respir Rev ;31(164)
Gundabolu K (2017) Anticoagulants could be a victim of Enzalutamide. J Oncol Pract 13(11):730–731
Al-Samkari H, Connors JM (2017) Anticoagulation and enzalutamide: caution over Convenience. J Oncol Pract 13(11):728–729
Shatzel JJ, Daughety MM, Olson SR, Beer TM, DeLoughery TG (2017) Management of anticoagulation in patients with prostate Cancer receiving Enzalutamide. J Oncol Pract 13(11):720–727
Bellet M, Ahmad F, Villanueva R et al (2019) Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication. Therapeutic Adv Med Oncol 11:1758835919833867
Gulikers JL, Slikkerveer M, Winckers K et al (2022) Case report: drug-drug interaction between alectinib and apixaban in NSCLC. Curr Probl Cancer: Case Rep 7:100186
Santini D, Citarella F, Vincenzi B, Russano M, Tonini G, Stellato M (2019) Cabozantinib and apixaban: an hitherto unreported interaction. Experimental Hematol Oncol 8(1):22
Kravchenko OV, Boyce RD, Gomez-Lumbreras A et al (2022) Drug–drug interaction between dexamethasone and direct-acting oral anticoagulants: a nested case–control study in the National COVID Cohort Collaborative (N3C). BMJ Open 12(12):e066846
Iyer GS, Tesfaye H, Khan NF, Zakoul H, Bykov K (2023) Trends in the use of oral anticoagulants for adults with venous thromboembolism in the US, 2010–2020. JAMA Netw Open 6(3):e234059–e234059
Kremers P (2002) In vitro tests for Predicting Drug-Drug interactions: the need for validated procedures. Pharmacol Toxicol 91(5):209–217
Puma Biotechnology Inc (2022) NERLYNX (neratinib) [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf. Accessed December 13, 2022
Dhopeshwarkar N, Yang W, Hennessy S, Rhodes JM, Cuker A, Leonard CE (2022) Bleeding with concomitant ibrutinib and oral anticoagulant therapy: A population-based cohort study. American Journal of Hematology ;n/a(n/a)
Ferri N, Colombo E, Tenconi M, Baldessin L, Corsini A (2022) Drug-drug interactions of direct oral anticoagulants (DOACs): from pharmacological to clinical practice. Pharmaceutics 14(6). https://doi.org/10.3390/pharmaceutics14061120
Tufano A, Galderisi M, Esposito L et al (2018) Anticancer Drug-Related Nonvalvular Atrial Fibrillation: Challenges in Management and Antithrombotic strategies. Semin Thromb Hemost 44(04):388–396
Acknowledgements
We thank Ms. Adelia Grabowsky, health sciences librarian at Auburn University, for her help in getting access to MedDRA dictionary version 25.0.
Funding
This research did not receive extramural funding from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
Truong: Conceptualization; Data curation; Formal analysis; Methodology; Writing - original draft. Hornsby: Methodology; Validation; Writing - review and editing. Fox: Methodology; Validation; Writing - review and editing. Chou: Methodology; Validation; Writing - review and editing. Zheng: Methodology; Validation; Writing - review and editing. Qian: Conceptualization; Methodology; Supervision; Validation; Writing - review and editing.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest to report for all authors.
Ethics approval
The study was granted an exemption by the Auburn University institutional review board (IRB), and the analysis was performed per the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient consent statement
not applicable.
Permission to reproduce material from other sources
not applicable.
Clinical trial registration
not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Truong, B., Hornsby, L., Fox, B.I. et al. Screening for clinically relevant drug-drug interactions between direct oral anticoagulants and antineoplastic agents: a pharmacovigilance approach. J Thromb Thrombolysis 56, 555–567 (2023). https://doi.org/10.1007/s11239-023-02879-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02879-7