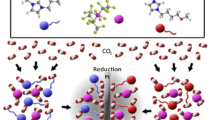
The effect of the nature of the solvent (DMF, DMSO, CH3CN, N-methylpyrrolidinone, THF) and supporting electrolyte (Bu4NBr, Et4NBr, Et4NClO4,Me4NBr, LiBF4, NaBF4, and KBF4) on the electrochemical activation and carboxylation of fluorine-containing aromatic imines by the action of CO2 was studied for the case of p-and m-fluorobenzylideneaniline. It was shown that factors promoting the formation of intimate ion pairs between the radical-anions of the imines – the initial products of the processes in the electrochemical activation of the imines – and the cations of the supporting electrolyte (decrease of the polarity of the medium and decrease of the radius of the cation in the supporting electrolyte) significantly reduce the effectiveness of electrochemical carboxylation right down to its complete cessation.

Similar content being viewed by others
References
V. G. Koshechko, V. E. Titov, V. N. Bondarenko, and V. D. Pokhodenko, J. Fluor. Chem., 129, 701-706 (2008).
V. N. Bondarenko, V. E. Titov, V. G. Koshechko, and V. D. Pokhodenko, Teor. Éksp. Khim., 44, No. 5, 265-271 (2008). [Theor. Exp. Chem., 44, No. 5, 271-277 (2008) (Engl. Transl.).]
V. D. Pokhodenko, A. A. Beloded, and V. G. Koshechko, Oxidation–Reduction Reactions of Free Radicals [in Russian], Naukova Dumka, Kiev (1977), p. 274.
Z. V. Todres, Ion-Radical Organic Chemistry: Principles and Applications, Marcel Dekker, New York (2003).
M. Schwarz, Ions and Ion Pairs in Organic Reactions [Russian translation], I. P. Beletskaya (ed.), Mir, Moscow (1975).
D. R. Burfield and R. H. Smithers, J. Org. Chem., 43, 3966-3968 (1978).
A. J. Gordon and R. A. Ford, The Chemist’s Companion [Russian translation], Mir, Moscow (1976), p. 439.
W. R. Fawcett, P. A. Forte, R. O. Loutfy, and J. M. Prokipcak, Can. J. Chem., 50, No. 2, 263-269 (1972).
T. Fukushima, T. Oyama, and M. Tomoi, React. Funct. Polym., 56, 59-73 (2003).
V. G. Koshechko and V. D. Pokhodenko, Teor. Éksp. Khim., 33, No. 5, 268-283 (1997). [Theor. Exp. Chem., 33, No. 5, 230-247 (1997) (Engl. Transl.).]
A. P. Tomilov, Élektrokhimiya, 32, No. 1, 30-41 (1996).
Ya. I. Gerasimov, et al. (eds.), Course of Physical Chemistry [in Russian], Vol. 2, Khimiya, Moscow (1973).
L. P. Hammett, Fundamentals of Physical Organic Chemistry [Russian translation], Mir, Moscow (1972).
Author information
Authors and Affiliations
Corresponding author
Additional information
Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 46, No. 1, pp. 8-13, January-February, 2010.
Rights and permissions
About this article
Cite this article
Titov, V.E., Bondarenko, V.N., Koshechko, V.G. et al. Effect of the nature of the solvent and the supporting electrolyte on the electrochemically activated insertion of CO2 into fluorine-containing aromatic imines with the production of amino acids. Theor Exp Chem 46, 8–13 (2010). https://doi.org/10.1007/s11237-010-9113-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-010-9113-6