Abstract
Integration of morphological and molecular approaches to species delineation has become an essential part of digenean trematode taxonomy, particularly when delimiting cryptic species. Here, we use an integrated approach to distinguish and describe two morphologically cryptic species of Hysterolecitha Linton, 1910 (Trematoda: Lecithasteridae) from fishes of Moreton Bay, Queensland, Australia. Morphological analyses of Hysterolecitha specimens from six fish species demonstrated a complete overlap in morphometric data with no reliable differences in their gross morphological characters that suggested the presence of more than one species. Distinctions in ITS2 rDNA and cox1 mtDNA sequence data for corresponding specimens suggested the presence of two forms. A principal component analysis on an imputed dataset showed clear separation between the two forms. These two forms are partially separated on the basis of their host’s identity. Therefore, we describe two morphologically cryptic species: Hysterolecitha melae n. sp. from three species of Abudefduf Forsskål and one species of Parma Günther (Pomacentridae), with the Bengal sergeant, Abudefduf bengalensis (Bloch), as the type-host; and Hysterolecitha phisoni n. sp. from species of Pomacentridae (including A. bengalensis), Pomatomidae and Siganidae, with the black rabbitfish, Siganus fuscescens (Houttuyn), as the type-host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hysterolecithinae Yamaguti, 1958 (Lecithasteridae) is the second largest of the six lecithasterid subfamilies, comprising four genera, Hysterolecitha Linton, 1910, Hysterolecithoides Yamaguti, 1934, Machidatrema León-Règagnon, 1998, and Thulinia Gibson & Bray, 1979. Members of the Hysterolecithinae differ from those of other lecithasterid subfamilies in the possession of Juel’s organ and a uterine seminal receptacle (Gibson, 2002). The type-genus, Hysterolecitha, is the richest of the four hysterolecithine genera, comprising 22 recognised marine and freshwater species. Species of this genus are distinguished from those of Hysterolecithoides, Machidatrema and Thulinia by an anterior fusion of the excretory ducts, absence of filamented eggs, and a weakly developed sinus-sac.
Species of Hysterolecitha have been reported from fishes from 21 families and most have been reported with oioxenous host-specificity (Table 1). Notably, while some species have been reported to be stenoxenic or euryxenic (infecting several host species of a single family or multiple families, respectively) or have extensive geographical distributions, these broad specificities and distributions have yet be confirmed with molecular data. Just two species of Hysterolecitha have been reported from Australian waters, H. heronensis Bray, Cribb & Barker, 1993 and H. nahaensis Yamaguti, 1942. Hysterolecitha heronensis was described from the Philippine damsel, Pomacentrus philippinus Evermann & Seale (Pomacentridae), off Heron Island in the southern Great Barrier Reef (GBR), Australia, and was reported from four other species of Pomacentrus Lacépède on the GBR (Bray et al., 1993; Barker et al., 1994; Sun et al., 2012). Hysterolecitha nahaensis was originally described from the humbug damselfish, Dascyllus aruanus (Linnaeus), off Okinawa, Japan (Yamaguti, 1942), and has since been reported mainly from pomacentrid fishes from the GBR (Bray et al., 1993; Barker et al., 1994; Sun et al., 2012) and the South China Sea (King, 1964; Zhokhov et al., 2018), and rarely from other fish families in localities such as the Celebes Sea (Yamaguti, 1953) and the Mozambique Channel (Parukhin, 1989).
Here, we describe two new species of Hysterolecitha from fishes of Moreton Bay, Queensland, Australia. These two new species are essentially cryptic relative to each other in that, despite being clearly distinct genetically while occurring in sympatry, they are effectively morphologically indistinguishable. Although these two species of Hysterolecitha infect an overlapping range of host species, they can be partially distinguished on the basis of their host range.
Materials and methods
Specimen collection
Fishes were collected from Moreton Bay in southeast Queensland, Australia between 2015 and 2021 via line-fishing and tunnel netting. Fishes were euthanised via an overdose of anaesthetic (AQUI-S®, AQUI-S New Zealand Ltd, Lower Hutt, New Zealand). The gastrointestinal tract was removed and examined for digeneans using the ‘gut wash’ method (Cribb & Bray, 2010). Digeneans were washed in saline, fixed in near-boiling saline, and preserved in 80% ethanol. Multiple specimens were prepared as hologenophores for parallel morphological and molecular analyses (Pleijel et al., 2008).
Morphological analyses
Specimens were rinsed with distilled water, overstained in Mayer’s haematoxylin, destained in a 1% hydrochloric acid solution, and neutralised in an 1% ammonium hydroxide solution. Specimens were then dehydrated in a graded series of ethanol solutions, cleared in methyl salicylate, and mounted in Canada balsam. Morphometric data were taken using a camera (Olympus SC50) mounted on a compound microscope (Olympus BX-53), and cellSens Standard imaging software. Measurements are in micrometres and are presented in Table 2. Drawings were made using a drawing tube attachment and digitised in Adobe Illustrator. Details of the two new species have been submitted to ZooBank and registered with Life Science Identifiers (LSID) to comply with the recommendations set out in the International Code of Zoological Nomenclature (ICZN, 2012). Specimens are lodged at the Queensland Museum (QM), Brisbane, Queensland, Australia.
For morphometric analyses, only characters from hologenophores with associated molecular data and paragenophores which were inferred as distinct based on host identity were used. The pre-ovarian length was used as a proxy for body length. To test the significance of some morphometric differences, Welch’s t-test was used. A principal component analysis (PCA) was used to further explore the morphometric dataset in R (R Core Team, 2022) using the packages ‘FactoMineR’ (Lê et al., 2008) for the analysis and ‘factoextra’ (Kassambara & Mundt, 2020) for visualisation. As some specimens were damaged or incomplete, not all characters could be measured or included in the analysis. Standard PCA methods are not suitable for incomplete datasets; in this study, deleting individuals or variables with incomplete observations would decrease an already limited dataset and would reduce the statistical power of the analysis. To address this, the package ‘mice’ (van Buuren & Groothuis-Oudshoorn, 2011) was used to impute the missing measurements; these missing values were predicted using multiple imputations based on fully conditional specification, where each variable (or character) is imputed by a separate model. Only specimens with at least 50% of the original measurements were included in the imputation and analysis (n = 28). To test the significance of the PCA, the R package ‘PCAtest’ (Camargo, 2022) was used with 1000 bootstrap replications and 1000 random permutations.
Molecular analysis
Genomic DNA was extracted using a standard phenol/chloroform extraction method (Sambrook & Russell, 2001) and sequence data were generated for one ribosomal DNA (rDNA) marker, the second internal transcribed spacer region (ITS2), and one mitochondrial DNA (mtDNA) marker, the cytochrome c oxidase subunit 1 (cox1). These regions were amplified using the primers 3S (5′-GGT ACC GGT GGA TCA CGT GGC TAG TG-3′, Morgan & Blair, 1995) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′, Cribb et al., 1998) for ITS2, and Dig_cox1Fa (5′-ATG ATW TTY TTY TTY YTD ATG CC-3′, Wee et al., 2017) and Dig_cox1R (5′-TCN GGR TGH CCR AAR AAY CAA AA-3′, Wee et al., 2017) for cox1. Amplification was conducted on a TaKaRa PCR Thermal Cycler (TP-690) and amplified DNA was sent to the Australian Genome Research Facility for purification and dual direction Sanger sequencing using the amplification primers.
The ITS2 rDNA and cox1 mtDNA datasets were aligned separately in MEGA X (Kumar et al., 2018) using MUSCLE (Edgar, 2004), with UPGMA clustering for iterations 1 and 2. The final dataset for the ITS2 rDNA alignment contained 559 base pairs. The cox1 mtDNA alignment was translated (echinoderm/flatworm mitochondrial code) and examined in Mesquite version 3.70 (Maddison & Maddison, 2021) for internal stop codons and to determine the correct reading frame. The alignment was trimmed after the correct reading frame was determined. All codon positions were then tested for non-stationarity in PAUP* version 4.0a (Swofford, 2003), and substitution saturation using the “Test of substitution saturation by Xia et al.” function (Xia et al., 2003; Xia & Lemey, 2009) implemented in DAMBE version 7.2 (Xia, 2018). Non-stationarity and substitution saturation were not detected, and as such, all codons were used in subsequent analyses. The final dataset for the cox1 alignment contained 474 base pairs. Neighbour-joining analyses were conducted for each alignment with the following parameters: “Test of Phylogeny = Bootstrap method”, “No. of Bootstrap Replications = 10,000”, “Model/Method = No. of differences”, “Substitutions to Include = d: Transitions + Transversions”, “Rates among Sites = Uniform rates” and “Gaps/Missing Data Treatment = Pairwise deletion”. Pairwise differences for each alignment were estimated using the following parameters: “Variance Estimation Method = None”, “Model/Method = No. of differences”, “Substitutions to Include = d: Transitions + Transversions”, “Rates among Sites = Uniform rates” and “Gaps/Missing Data Treatment = Pairwise deletion”.
Results
Overview
Specimens morphologically consistent with the genus Hysterolecitha were collected from four pomacentrid species, the Bengal sergeant, Abudefduf bengalensis (Bloch), the Indo-Pacific sergeant, A. vaigiensis (Quoy & Gaimard), Whitley’s sergeant, A. whitleyi Allen & Robertson, and the bigscale scaly fin, Parma oligolepis Whitley, one pomatomid species, the tailor, Pomatomus saltatrix (Linnaeus), and one siganid species, the black rabbitfish, Siganus fuscescens (Houttuyn). Initial morphological examination suggested that the collection represented a single species; however, the molecular data demonstrated the clear presence of two forms (Figure 1). A total of 38 ITS2 rDNA and 38 cox1 mtDNA sequences were generated. From the ITS2 sequence data, two forms were recognised, differing at 21 base positions. Corresponding cox1 sequence data differed at 89–91 base positions. Both forms showed intraspecific variation in the cox1 region, with one varying at a single base position, and the second at 1–7 base positions. Re-examination of hologenophores revealed some marginally diagnostic characters that could be used (albeit unreliably) to distinguish between the two forms, specifically the forebody length relative to the body length or the pre-ovarian length, and the development of the terminal genitalia. The two forms are partly biologically distinguished by their host; that is, siganid and pomatomid hosts were only infected by one form, whereas one pomacentrid, A. bengalensis, was infected by both.
The PCA of the morphometric data revealed a combination of characters which separated the two forms (Figure 2 and Table 2). The PCA permutation tests revealed significant ψ (129.027, p = 0) and φ (0.378, p <.001) values, indicating that the PCA was biologically significant. Based on cumulative variance and eigenvalues, the first five to eight principal components (PCs) would be retained for further analyses as they accounted for at least 80% of the total variation and had eigenvalues above one (Table 3). However, only PC1 and PC2 were statistically significant and were retained for subsequent analyses. Based on the loading values, a combination of 22 variables contributed significantly to PC1, explaining 35.4% of the total variation (95% confidence interval 30.2–44.8; p <.001) and a combination of six variables contributed significantly to PC2, explaining 14.9% of the total variation (95% confidence interval 12.3–21.9; p <.001). For list of significant variables see Table 2.
The integrated analysis of the morphological and molecular data (as well as the host-specificity) suggest that the two forms of Hysterolecitha in the collection represent two species. The forms do not agree with known species of Hysterolecitha and are described as new herein.
Family Lecithasteridae Odhner, 1905
Genus Hysterolecitha Linton, 1910
Type-species Hysterolecitha rosea Linton, 1910 (type by original designation)
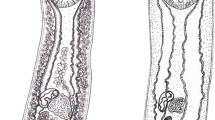
Hysterolecitha melae n. sp. (Figures 3a–b and 4a)
Adult specimens of Hysterolecitha melae n. sp. and H. phisoni n. sp. collected from Moreton Bay, Australia. a, b, Hysterolecitha melae n. sp. from Abudefduf bengalensis (Bloch), (a) hologenophore, (b) paragenophore, ventral view. c, d, H. phisoni n. sp. from Siganus fuscescens (Houttuyn), (c) hologenophore, (d) paragenophore, ventral view. Scale-bars: 200 µm.
Terminal genitalia of Hysterolecitha melae n. sp. and H. phisoni n. sp. collected from Moreton Bay, Australia. a, H. melae n. sp. from Abudefduf bengalensis (Bloch), ventral view. b, H. phisoni n. sp. from Siganus fuscescens (Houttuyn), ventral view. c, H. phisoni n. sp. from A. bengalensis, ventral view. Scale-bars: 100 µm.
Type-host: Abudefduf bengalensis (Bloch), Bengal sergeant (Pomacentridae).
Type-locality: Off Amity Point (27°24′ S 153°25′ E), Moreton Bay, Queensland, Australia.
Site in host: Stomach.
Other hosts: Abudefduf vaigiensis (Quoy & Gaimard), Indo-Pacific sergeant; Abudefduf whitleyi Allen & Robertson, Whitley’s sergeant; Parma oligolepis Whitley, Bigscale scaly fin (Pomacentridae).
Prevalence: 21 of 60 A. bengalensis; 7 of 18 A. vaigiensis; 8 of 22 A. whitleyi; 1 of 2 P. oligolepis.
Type-material: Holotype (hologenophore, QM G240369), and 16 paratypes (10 hologenophores, QM G240370–74, G240380–83, G240385; six paragenophores, QM G240375-79, G240384).
Representative DNA sequences: ITS2 rDNA, 30 identical sequences, four (one of each host/locality combination) submitted to GenBank; cox1 mtDNA, 29 sequences, five (one sequence of each genotype/host/locality combination) submitted to GenBank (see Table 4 for accession numbers).
ZooBank registration:urn:lsid:zoobank.org:act:222B5282-1B30-4E90-82AD-FED139E6C0EB.
Etymology: This new species is named for the first author’s sister, Melissa ‘Mel’ Duong, in recognition of her constant support and encouragement.
Description
Based on 19 hologenophores and eight paragenophores (from all hosts; see Table 2 for measurements). Body elongate, cylindrical, with hindbody wider than forebody, widest at level of mid-ventral sucker. Anterior end of body rounded, tapering distally. Posterior end rounded. Pre-oral lobe usually distinct. Oral sucker globular, subterminal, with anterior half generally broader than posterior half. Ventral sucker rounded, larger than oral sucker, with small papilla-like protrusions on internal surface. Pharynx subglobular, slightly wider than long, overlaps oral sucker dorsally. Oesophagus shorter than pharynx. Intestinal bifurcation in mid-forebody. Caeca irregularly narrow, dorsal to uterus, testes, ovary and vitellarium, reach close to posterior extremity. Genital pore a transverse ellipse, median. Sinus-sac claviform, longer than wide, proximal end typically borders anterior margin of ventral sucker, encloses well-developed sinus-organ. Pars prostatica oval, represented by cluster of prostatic cells. Seminal vesicle saccular, elongate, extends to mid-level of ventral sucker. Testes irregularly oval, oblique, generally separated, in anterior half of hindbody. Ovary transversely oval, in posterior half of hindbody. Seminal receptacle rounded, post-ovarian, typically obscured by vitellarium lobes, uterus or eggs. Juel’s organ not detected. Uterus fills most of hindbody; coils typically do not extend anteriorly past posterior margin of ventral sucker. Metraterm not differentiated from uterus. Eggs numerous, small, tanned, operculate, without bipolar filaments, tightly packed together in uterine coils. Vitellarium comprised of seven to eight compact digitiform lobes, radiating from central point immediately posterior to ovary. Excretory arms tubular, typically obscured by uterus and eggs, unite dorsally at level of pharynx. Excretory pore terminal.
Hysterolecitha phisoni n. sp. (Figures 3c–d and 4b–c)
Type-host: Siganus fuscescens (Houttuyn), Black rabbitfish (Siganidae).
Type-locality: Off Green Island (27°25′ S 153°14′ E), Moreton Bay, Queensland, Australia.
Site in host: Stomach.
Other hosts: Abudefduf bengalensis (Bloch), Bengal sergeant (Pomacentridae); Pomatomus saltatrix (Linnaeus), Tailor (Pomatomidae).
Other localities: Off Garden Island (27°37′ S 153°20′ E) and off Amity Point (27°24′ S 153°25′ E), Moreton Bay, Queensland, Australia.
Prevalence: 3 of 60 A. bengalensis; 1 of 18 P. saltatrix; 6 of 31 S. fuscescens.
Type-material: Holotype (QM G240386), and 10 paratypes (five hologenophores, QM G240387–88, G240393–94, G240396; five paragenophores, QM G240389–92, G240395).
Representative DNA sequences: ITS2 rDNA, eight identical sequences, three (one of each host/locality combination) submitted to GenBank; cox1 mtDNA, nine sequences, six (one sequence of each genotype/host/locality combination) submitted to GenBank (see Table 4 for accession numbers).
ZooBank registration: urn:lsid:zoobank.org:act:4BC2369B-5BAF-4016-A16C-BB20E1526A09.
Etymology: The new species is named for the first author’s partner, Brody Phi Son Ly, in recognition of his constant support and encouragement.
Description
Based on five hologenophores (from A. bengalensis and S. fuscescens) and nine paragenophores (from S. fuscescens; see Table 2 for measurements). Body elongate, cylindrical, widest at level of mid-ventral sucker. Anterior end of body rounded, slightly tapering distally. Posterior end rounded, blunt. Pre-oral lobe usually distinct. Oral sucker globular, subterminal, with anterior half generally broader than posterior half. Ventral sucker in proximal region of anterior half of body, rounded, larger than oral sucker, with small papilla-like protrusions on internal surface. Pharynx subglobular, slightly longer than wide, overlaps oral sucker dorsally. Oesophagus shorter than pharynx. Intestinal bifurcation in mid-forebody. Caeca irregularly narrow or wide, dorsal to uterus, testes, ovary and vitellarium, reach close to posterior extremity. Genital pore a transverse ellipse, median. Sinus-sac rounded, generally slightly longer than wide, encloses poorly developed sinus-organ. Pars prostatica oval or reniform, lined by prostatic cells. Seminal vesicle saccular, dorsally overlaps anterior margin of ventral sucker. Testes oval, oblique, generally contiguous, in anterior half of hindbody. Ovary transversely oval, in posterior half of hindbody. Juel’s organ and seminal receptacle not detected. Uterus fills most of hindbody; coils can extend dorsally past posterior margin of ventral sucker. Metraterm not differentiated from uterus. Eggs numerous, small, tanned, operculate, without bipolar filaments. Vitellarium comprised of seven to eleven compact digitiform lobes, radiating from central point immediately posterior to ovary. Excretory arms tubular, typically obscured by uterus and eggs, unite dorsally at level of pharynx. Excretory pore terminal.
Remarks
The current collection of Hysterolecitha specimens sampled from Moreton Bay fishes represents a case of sympatric, cryptic species that are associated with a partial overlap in host species ranges. Representative specimens from each individual host were prepared as a hologenophore and the subsequent genetic data associated with each hologenophore was used to tentatively identify paragenophores. This process resulted in collections of worms from single host individuals being identified as the same species. As the two species are essentially cryptic, we acknowledge that this division may not be completely reliable; definitive species identification is presently dependent on genetic data (and, partly, host identity). Based on this genetic delineation, specimens were compared morphologically to find a basis of distinction. There is a significant difference in the forebody length relative to: a) body length and b) pre-ovarian length between specimens of H. melae n. sp. (a: Mean, M = 20.7, Standard Deviation, SD = 2.4; b: M = 29.6, SD = 4) and H. phisoni n. sp. (a: M = 28.1, SD = 2.6; b: M = 38, SD = 3.3); a: t(13) = -6.369, p <.001, b: t(31) = -6.676, p <.001. Specimens of H. melae n. sp. generally have shorter forebodies relative to their body length and pre-ovarian length, whereas H. phisoni n. sp. specimens generally have longer forebodies; however, this pattern is not completely consistent and is not observed across all specimens (Figure 5). The specimens also differed in the development of their terminal genitalia; H. melae n. sp. generally has a well-developed sinus-organ and a smaller pars prostatica than H. phisoni n. sp. which has a poorly developed sinus-sac and a larger pars prostatica.
Discussion
Species delineation
Bray et al. (2022) proposed a set of criteria for trematode species delineation which requires reciprocal monophyly in the most discriminatory molecular markers in combination with either morphological differences relative to other taxa or distinctions in host species ranges relative to those of closely related taxa. The recognition of two species of Hysterolecitha here is based on ITS2 and cox1 sequence data, and morphometric analyses that showed that the specimens formed distinct clusters. The strong reciprocal monophyly is the key evidence in justifying the recognition of two species. The two species are also partly distinguished by host range; specimens of H. melae n. sp. were found in only four pomacentrid species whereas H. phisoni n. sp. was found commonly in a siganid, uncommonly in a pomacentrid, and rarely in a pomatomid. Therefore, the interpretation of these data is that the present collection of Hysterolecitha from Moreton Bay comprises two genetically distinct species that are essentially morphologically cryptic.
Relative to the 20 marine species of Hysterolecitha, H. melae n. sp. and H. phisoni n. sp. are morphologically most similar to H. heronensis (reported from pomacentrids), H. lintoni Srivastava, 1939 (reported from an ariid), H. nahaensis (reported from several families including pomacentrids and siganids), H. rosea Linton, 1910 (reported mostly from acanthurids), and H. teuthis Nagaty, 1956 (reported from a siganid). However, both the new species differ from H. heronensis by a having smaller sucker width ratio (1:1.8–2.3 and 1:1.8–2.0 vs 1:2.65), a short sinus-sac (vs elongated) and compact irregular digitiform vitelline lobes (vs elongated digitiform lobes) and from H. lintoni by having an oesophagus present (vs absent) and a saccular seminal vesicle (vs constricted). The two new species differ from H. nahaensis by having irregular digitiform vitelline lobes (vs rounded lobes), and from H. rosea and H. teuthis by having a saccular seminal vesicle (vs sinuous). Of the remaining species of Hysterolecitha, the two new species differ from H. acanthuri Annereaux, 1947, H. palani Yamaguti, 1970, H. sogandaresi Nahhas & Cable, 1964, and H. trilocalis King & Noble, 1961 in having a saccular seminal vesicle (vs sinuous), from H. arii Wang, 1982, H. blepsiae Layman, 1930, and H. vitellograndis (Layman, 1930) Skrjabin & Guschanskaja, 1954 in having digitiform vitelline lobes (vs rounded or club-shaped), and from H. brasiliensis de Oliveira, Amato & Knoff, 1988, H. crassivesiculata Bravo-Hollis, 1956, H. flaticaudata Bilqees, Feroze & Shaukat, 2004, and H. indonesiana Machida, 1996 in having smaller eggs (19–27 × 7–11 vs 24–43 × 16–22, 34–40 × 18–22, 34–42 × 21–31 and 32–39 × 18–22, respectively). The two new species differ from H. chirocentri Ku & Shen, 1964 in possessing a united vitellarium (vs divided into two clusters), from H. elongata Manter, 1931 in having a shorter post-ovarian region (vs elongated), and from H. progonimus Ku & Shen, 1964 in having a seminal vesicle that dorsally overlaps the anterior end of the ventral sucker (vs a seminal vesicle that terminates at the level of the mid-forebody). Finally, the two new species differ from H. soniae León-Règagnon, Perez-Ponce de Leon & Lamothe-Argumedo, 1997 in possessing an oval pars prostatica (vs sinuous).
The genetic differences reported here for H. melae n. sp. and H. phisoni n. sp. are generally comparable to other combinations of cryptic species. Recent studies have reported genetic differences of up to 21 base positions in the ITS2 region and up to 53 base positions in the cox1 region for cryptic species of lepocreadiids from the GBR (Bray et al., 2018; Bray et al., 2022) and monorchiids from the GBR and Japan (Wee et al., 2022). However, the genetic differences among some closely related, non-cryptic species has been reported to be much lower. For example, studies on morphologically distinguishable species of bivesiculids (Trieu et al., 2015; Cribb et al., 2022) and lepocreadiids (Bray et al., 2018; Bray et al., 2022) from GBR fishes have reported differences at one to two base positions in the ITS2 region and up to 52 base positions in the cox1 region. We conclude that use of a ‘yardstick’ approach to the interpretation of the significance of levels of molecular distinction is problematic. Instead, interpretations are best made on a case by case basis in the light of all available evidence. Here, we interpret the evidence as clearly indicating the presence of two species.
The genus Hysterolecitha
Previous reports of species of Hysterolecitha have been overwhelmingly based on morphometric data in isolation. A search for Hysterolecitha sequence data on GenBank returned only a single result, an 18S sequence of H. nahaensis (from an unknown host and locality), generated as part of a large phylogenetic study of the Hemiuroidea (Blair et al., 1998). This lack of genetic data associated with reports (and descriptions) makes reliable species delineation and identification difficult, especially for species as morphologically cryptic as H. melae n. sp. and H. phisoni n. sp. Without supporting genetic data, the current collection of Moreton Bay Hysterolecitha specimens would certainly have been considered a single euryxenous species with marginal intraspecific morphological variation. The genetic data, however, clearly indicates that the new specimens comprise two species with different forms of host-specificity (euryxenous and stenoxenous).
Of the known marine Hysterolecitha species, five have been reported from more than one locality, with H. nahaensis [see Yamaguti (1942), Yamaguti (1953), Parukhin (1989) and Zhokhov et al. (2018)] and H. rosea [see Linton (1910) and Wang (1982)] being reported as the most widespread. However, like the majority of Hysterolecitha species, the two new species are known from only a single locality, but unlike for most species of the genus, there is some evidence of absence. Hysterolecitha melae n. sp. and H. phisoni n. sp. have not been detected at other Australian localities, specifically the GBR, where two other known species (H. heronensis and H. nahaensis) have been found (Bray et al., 1993; Barker et al., 1994; Sun et al., 2012). While the distribution of Pomatomus saltatrix does not indicate it would be found in the GBR (Bray, 2022), the pomacentrid and siganid hosts of H. melae n. sp. and H. phisoni n. sp. have broad distributions that encompass the GBR (Randall et al., 1998; Parmentier & Frédérich, 2016; Bray, 2020). However, based on extensive collecting in Queensland waters for over 20 years, we have not detected either of the two new species (or the known species) outside of their respective known localities. That is, H. melae n. sp. and H. phisoni n. sp. have not been found in the GBR, and H. heronensis and H. nahaensis have not been found from Moreton Bay, despite the presence of suitable hosts for each species at these locations.
Cryptic species complexes in the Hemiuroidea
Digeneans have been reported to have some of the highest levels of cryptic diversity relative to other helminth taxa (Pérez-Ponce de León & Poulin, 2018). The findings of cryptic diversity here extend a growing list of cases within the Hemiuroidea. One reported case of cryptic hemiuroids was by Carreras-Aubets et al. (2011) who described a new species related to the lecithasterid Aponurus laguncula Looss, 1907. The new species was recognised for combined molecular, morphological and host distinctions. The new species was not strictly morphologically cryptic relative to A. laguncula, but it was sufficiently inconspicuous to have escaped recognition for over 100 years since the description of A. laguncula, although that species was suspected to constitute a species complex due to its euryxenous host-specificity and wide geographical distribution (Bray & MacKenzie, 1990; Bray et al., 1993). Another case was for the genus Hirudinella de Blainville, 1828 (Hirudinellidae). For this genus, the delineation of species is made challenging by the huge size of the specimens and the lack of reliable characters to separate species (Gibson & Bray, 1979); as a result, many nominal Hirudinella species are no longer recognised. Calhoun et al. (2013) used ITS1, ITS2 and 28S rDNA sequence data to show that specimens consistent with Hirudinella ventricosa (Pallas, 1774) Baird, 1853 comprised four species. Despite the clarity of the molecular data, only two of the species (H. ventricosa and H. ahi Yamaguti, 1970) could be formally recognised; two other species were not named due to limitations associated with the morphological features and additional genetic data. Recent molecular work on morphologically similar specimens of Lecithaster Lühe, 1901 (Lecithasteridae) collected from fishes belonging to multiple families has resulted in the recognition of two species that had been synonymised principally on morphometric similarity (Atopkin et al., 2020). Lecithaster sayori Yamaguti, 1934 and L. salmonis Yamaguti, 1934 [previously synonymised with L. stellatus Looss, 1907 (see Manter & Pritchard, 1960) and L. gibbosus (Rudolphi, 1802) Lühe, 1901 (see Margolis & Boyce, 1969), respectively], were shown to be genetically distinct based on ribosomal markers. Most recently, adult and cercarial specimens morphologically consistent with Derogenes varicus (Müller, 1784) Looss, 1901 (Derogenidae) were shown to be genetically distinct, forming up to four lineages based on ribosomal and mitochondrial markers that were associated with different hosts and localities (Olson et al., 2003; Sokolov et al., 2021; Krupenko et al., 2022). Although the genetic distinctions are clear, the lack of corresponding adult morphological specimens has hindered the naming of these lineages as new species. The current findings of cryptic species of Hysterolecitha here are broadly consistent with the previous studies reviewed above. As is frequently the case, the distinct species here were largely associated with different host taxa. The most difficult problem of cryptic species, where combinations of species occur in the same host and the same locality (as partly occurred here), is not commonly reported.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Annereaux, R. F. (1947). Two new trematodes from Philippine fishes. Transactions of the American Microscopical Society, 66, 172–175.
Atopkin, D. M., Nakao, M., Besprozvannykh, V. V., Ha, N. D., Nguyen, H. V., & Sasaki, M. (2020). Morphological and molecular data for species of Lecithaster Lühe, 1901 and Hysterolecithoides Yamaguti, 1934 (Digenea: Lecithasteridae) from fish of East Asia and phylogenetic relationships within the Hemiuroidea Looss, 1899. Journal of Helminthology, 94, E14.
Barker, S. C., Cribb, T. H., Bray, R. A., & Adlard, R. D. (1994). Host-parasite associations on a coral reef: pomacentrid fishes and digenean trematodes. International Journal for Parasitology, 24, 643–647.
Bilqees, F. M., Feroze, R., & Shaukat, N. (2004). Hysterolecitha flaticaudata n. sp. (Digenea: Hemiuridae: Hysterolecithinae) from the fish Engraulis purava of Karachi Coast. Proceedings of Parasitology, 38, 95–98.
Blair, D., Bray, R. A., & Barker, S. C. (1998). Molecules and morphology in phylogenetic studies of the Hemiuroidea (Digenea: Treamtoda: Platyhelminthes). Molecular Phylogenetics and Evolution, 9, 15–25.
Bravo-Hollis, M. (1956). Trematodos de peces marinos de aguas Mexicanas. XI. Estudio de 17 digeneos de las costas del Pacifico, incluyendo seis especies nuevas y un genero nuevo. Anales del Instituto de Biologia Universidad de Mexico, 27, 245–275.
Bray, D. J. (2020). Siganus fuscescens in Fishes of Australia. Available from: https://fishesofaustralia.net.au/home/species/4734
Bray, D. J. (2022). Pomatomus saltatrix in Fishes of Australia. Available from: https://fishesofaustralia.net.au/home/species/4246
Bray, R. A., Cribb, T. H., & Barker, S. C. (1993). The Hemiuroidea (Digenea) of pomacentrid fishes (Perciformes) from Heron Island, Queensland, Australia. Systematic Parasitology, 24, 159–184.
Bray, R. A., Cutmore, S. C., & Cribb, T. H. (2018). Lepotrema Ozaki, 1932 (Lepocreadidae: Digenea) from Indo-Pacific fishes, with the description of eight new species, characterised by morphometric and molecular features. Systematic Parasitology, 95, 693–741.
Bray, R. A., Cutmore, S. C., & Cribb, T. H. (2022). A paradigm for the recognition of cryptic trematode species in tropical Indo-West Pacific fishes: the problematic genus Preptetos (Trematoda: Lepocreadiidae) as a test-case. International Journal for Parasitology, 52, 169–203.
Bray, R. A., & MacKenzie, K. (1990). Aponurus laguncula Looss, 1907 (Digenea: Lecithasteridae): a report from herring, Clupea harengus L., in the eastern English Channel and a review of its biology. Systematic Parasitology, 17, 115–124.
Calhoun, D. M., Curran, S. S., Pullis, E. E., Provaznik, J. M., & Franks, J. S. (2013). Hirudinella ventricosa (Pallas, 1774) Baird, 1853 represents a species complex based on ribosomal DNA. Systematic Parasitology, 86, 197–208.
Camargo, A. (2022). PCAtest: Testing the statistical significance of Principal Component Analysis in R. PeerJ, 10:e12967. Available from: https://doi.org/10.7717/peerj.12967
Carreras-Aubets, M., Repullés-Albelda, A., Kostadinova, A., & Carrassón, M. (2011). A new cryptic species of Aponurus Looss, 1907 (Digenea: Lecithasteridae) from Mediterranean goatfish (Teleostei: Mullidae). Systematic Parasitology, 79, 145–159.
Chauhan, B. S. (1953). Studies on the trematode fauna of India. Part IV. Subclass Digenea (Prosostomata) (A revision of Hemiuroidea from the Indian region). Records of the Indian Museum, 51, 289–393.
Cribb, T. H., Anderson, G. R., Adlard, R. D., & Bray, R. A. (1998). A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). International Journal for Parasitology, 28, 1791–1795.
Cribb, T. H., & Bray, R. A. (2010). Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7.
Cribb, T. H., Bray, R. A., Justine, J.-L., Reimer, J., Sasal, P., Shirakashi, S., & Cutmore, S. C. (2022). A world of taxonomic pain: cryptic species, inexplicable host-specificity, and host-induced morphological variation among species of Bivesicula Yamaguti, 1934 (Trematoda: Bivesiculidae) from Indo-Pacific Holocentridae, Muraenidae and Serranidae. Parasitology, 149, 831–853.
de Oliveira, E. F., Amato, J. F. R., & Knoff, M. (1988). Hysterolecitha (Trematoda: Hemiuridae) from the mullet, Mugil liza, in the State of Rio de Janeiro, Brasil. Proceedings of the Helminthological Society of Washington, 55, 58–61.
Dyer, W. G., Williams, E. H., & Bunkley-Williams, L. (1992). Homalometron dowgialloi sp. n. (Homalometridae) from Haemulon flavolineatum and additional records of digenetic trematodes of marine fishes in the West Indies. Journal of the Helminthological Society of Washington, 59, 182–189.
Dyer, W. G., Williams, E. H., & Williams, L. B. (1985). Digenetic trematodes of marine fishes of the western and southwestern coasts of Puerto Rico. Proceedings of the Helminthological Society of Washington, 52, 85–94.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797.
Fischthal, J. H. (1977). Some digenetic trematodes of marine fishes from the Barrier Reef and Reef Lagoon of Belize. Zoologica Scripta, 6, 81–88.
Gibson, D. I. (2002). Family Lecithasteridae Odhner, 1905. In: Gibson, D. I., Jones, A. & Bray, R. A. (Eds) Keys to the Trematoda. London: CAB International and The Natural History Museum.
Gibson, D. I., & Bray, R. A. (1979). The Hemiuroidea: terminology, systematics and evolution. Bulletin of the British Museum (Natural History), 36, 35–146.
Gomes, D. C., de Fabio, S. P., & Rolas, F. J. T.-S. (1974). Contribuição para o conhecimento dos parasitos de peixes do litoral do Estado da Guanabara - parte II. Memórias do Instituto Oswaldo Cruz, 72, 9–19.
Ichihara, A. (1974). Hemiurid trematodes from marine fishes near the Tsushima Islands in the Sea of Japan. Proceedings of the Third International Congress of Parasitology (Munich), p. 1614–1615.
ICZN (2012). International Commission on Zoological Nomenclature: amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Kassambara, A., & Mundt, F. (2020). factoextra: extract and visualise the results of multivariate data analyses. Version 1.0.7. Available from: https://CRAN.R-project.org/package=factoextra
King, R. E. (1964). Three hemiurid trematodes from South Viet Nam. Transactions of the American Microscopical Society, 83, 435–439.
King, R. E., & Noble, E. R. (1961). A new species of Hysterolecitha (Trematoda: Hemiuridae) from the mudsucker Gillichthys mirabilis Cooper. Journal of Parasitology, 47, 465–468.
Knoff, M., Luque, J. L., & Amato, J. F. R. (1997). Community ecology of the metazoan parasites of grey mullets, Mugil platanus (Osteichthyes: Mugilidae) from the littoral of the State of Rio de Janeiro, Brazil. Revista Brasileira de Biologia, 57, 441–454.
Krupenko, D., Kremnev, G., Gonchar, A., Uryadova, A., Miroliubov, A., Krapivin, V., Skobkina, O., Gubler, A., & Knyazeva, O. (2022). Species complexes and life cycles of digenetic trematodes from the family Derogenidae. Parasitology, 149, 1590–1606.
Ku, C. T., & Shen, J. W. (1964). Studies on two new species of hemiurid-trematode, belonging to the genus Hysterolecitha Linton, 1910. Acta Scientarum Naturalium University Nankaiensis, 5, 37–43.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
Kuramochi, T. (2006). Digenetic trematodes of fishes caught in the Sagami Sea, Central Japan. Memoirs of the National Science Museum, Tokyo, 40, 175–186.
Layman, E. M. (1930). Parasitic worms from the fishes of Peter The Great Bay. Izvestiya Tikhookeanskoi Nauchno-Promyslovi Ostantsii, 3, 1–120.
Lê, S., Josse, J., & Husson, F. (2008). FactoMineR: an R package for multivariate analysis. Journal of Statistical Software, 25, 1–18.
León-Règagnon, V., Pérez-Ponce de León, G., & Lamothe-Argumedo, R. (1997). Hemiuriformes de peces marinos de la Bahia de Chamela, Mexico, con la descripcion de una nueva especie del genero Hysterolecitha (Digenea: Hemiuridae: Lecithasterinae). Anales del Instituto de Biología, Universidad Nacional Autónoma de México (Series Zoología), 66, 1–34.
Li, Q. K., Qiu, Z. Z., & Zhang, R. S. (1989). Digenetic trematodes of fishes from the Bo-Hai Sea, China VI (Trematoda: Opecoelidae). Acta Zootaxonomica Sinica, 1, 12–16.
Linton, E. (1910). Helminth fauna of the Dry Tortugas. II. Trematodes. Papers from the Tortugas Laboratory of the Carnegie Institute of Washington, 4, 11–98.
Machida, M. (1996). Digenean trematodes from mullets in Japanese and adjacent waters. Japanese Journal of Parasitology, 45, 123–133.
Maddison, W. P., & Maddison, D. R. (2021). Mesquite: a modular system for evolutionary analysis. Version 3.70. Available from: https://www.mesquiteproject.org
Manter, H. W. (1931). Some digenetic trematodes of marine fishes of Beaufort, North Carolina. Parasitology, 34, 396–411.
Manter, H. W. (1947). The digenetic trematodes of marine fishes of Tortugas. American Midland Naturalist, 38, 257–416.
Manter, H. W., & Pritchard, M. H. (1960). Additional hemiurid trematodes from Hawaiian fishes. Proceedings of the Helminthological Society of Washington, 27, 165–180.
Margolis, L., & Boyce, N. P. (1969). Life span, maturation, and growth of two hemiurid trematodes, Tubuolovescila lindbergi and Lecithaster gibbosus, in Pacific salmon (genus Oncorhynchus). Journal of the Fisheries Research Board of Canada, 26, 893–907.
Mehra, R. K. (1969). A new species of the genus Hysterolecitha Linton, 1910 (Trematoda: Hemiuridae) from Ophiocephalus punctatus (Bloch.). Annual Number National Academy of Sciences India, 1961, 83–84.
Mehra, R. K., Kharoo, V. K., & Dhar, R. L. (1985). Studies on the history of genus Hysterolecitha Linton, 1910 with the description of Hysterolecitha ophiocephali from a freshwater fish Ophiocephalus punctatus from Allahabad. Indian Journal of Helminthology, 36, 26–31.
Morgan, J. A., & Blair, D. (1995). Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology, 111, 609–615.
Nagaty, H. F. (1956). Trematodes of fishes from the Red Sea. Part 6. On five distomes including one new genus and four new species. Journal of Parasitology, 42, 151–155.
Nahhas, F. M., & Cable, R. M. (1964). Digenetic and aspidogastrid trematodes from marine fishes of Curacao and Jamaica. Tulane Studies in Zoology, 11, 169–228.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Overstreet, R. M. (1973). Some species of Lecithaster Lühe, 1901 (Digenea: Hemiuridae) and related genera from fishes in the northern Gulf of Mexico. Transactions of the American Microscopical Society, 92, 231–240.
Parmentier, E., & Frédérich, B. (2016). Meet the damselfishes. In: Parmentier, E. & Frédérich, B. (Eds) Biology of Damselfishes. Boca Raton: CRC Press LLC.
Parukhin, A. M. (1976). Trematodes of fish in the Indian Ocean. Biologiya Morya, Kiev, 38, 76–84.
Parukhin, A. M. (1989). Parasitic worms of bottom fishes of the southern seas. Kiev: Naukova Dumka.
Pearse, A. S. (1949). Observations on flatworms and nemerteans collected at Beaufort, N.C. Proceedings of the U.S. National Museum, 100, 25–38.
Pérez-Ponce de León, G., & Poulin, R. (2018). An updated look at the uneven distribution of cryptic diversity among parasitic helminths. Journal of Helminthology, 92, 197–202.
Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., Sundberg, P., & Thollesson, M. (2008). Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371.
R Core Team. 2022. R: A language and environment for statistical computing. Available from: https://www.R-project.org/
Randall, J. E., Allen, G. R., & Steene, R. C. (1998). Fishes of the Great Barrier Reef and Coral Sea. USA: University of Hawaii Press.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: a laboratory manual. United States: Cold Spring Harbor Laboratory Press.
Shen, J. W. (1990). Digenetic trematodes of marine fishes from Hainan Island. Beijing: Science Press.
Shen, J. W., & Qiu, Z. Z. (1995). Studies on the trematodes of fishes from the Yellow Sea and the Bo Hai Sea. Beijing: Science Press.
Siddiqi, A. H., & Cable, R. M. (1960). Digenetic trematodes of marine fishes of Puerto Rico. Scientific Survey of Porto Rico and the Virgin Islands, 17, 257–369.
Sogandares-Bernal, F. (1959). Digenetic trematodes of marine fishes from the Gulf of Panama and Bimini, British West Indies. Tulane Studies in Zoology, 7, 69–117.
Sokolov, S. G., Atopkin, D. M., & Gordeev, I. I. (2021). Phylogenetic position of the hemiuroid genus Paraccacladium Bray & Gibson, 1977 (Trematoda: Hemiuroidea) and the status of the subfamily Paraccacladiinae Bray & Gibson, 1977. Marine Biology Research, 17, 31–40.
Srivastava, H. D. (1939). A new parasite of the genus Hysterolecitha Linton, 1910. Indian Journal of Veterinary Science, 9, 73–76.
Sun, D., Blomberg, S. P., Cribb, T. H., McCormick, M. I., & Grutter, A. S. (2012). The effects of parasites on the early life stages of a damselfish. Coral Reefs, 31, 1065–1075.
Swofford, D. L. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Available from: https://paup.phylosolutions.com/
Travassos, L., Teixeira de Freitas, J. F., & Bührnheim, P. F. (1967). Relatorio da excursao do Instituto Oswaldo Cruz ao Estado do Espirito Santo em novembro de 1964. Boletim do Museu de Biologia Mello Leitão, Zoologia, 31, 1–21.
Trieu, N., Cutmore, S. C., Miller, T. L., & Cribb, T. H. (2015). A species pair of Bivesicula Yamaguti, 1934 (Trematoda: Bivesiculidae) in unrelated Great Barrier Reef fishes: implications for the basis of speciation in coral reef fish trematodes. Systematic Parasitology, 91, 231–239.
van Buuren, S., & Groothuis-Oudshoorn, K. (2011). mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software, 43, 1–67.
Vigueras, I. P. (1958). Contribucion al conocimiento de la fauna helminthologica Cubana. Memorias de la Sociedad Cubana de Historia Natural Felipe Poey, 24, 17–38.
Wang, P. Q. (1982). Hemiuroid trematodes of marine fishes from Fujian Province, China. Journal of Fujian Teachers University (Natural Science), 2, 67–80.
Wee, N. Q.-X., Cribb, T. H., Shirakashi, S., & Cutmore, S. C. (2022). Three new species of Helicometroides Yamaguti, 1934 from Japan and Australia, with new molecular evidence of a widespread species. Parasitology, 149, 622–639.
Wee, N. Q.-X., Cribb, T. H., Bray, R. A., & Cutmore, S. C. (2017). Two known and one new species of Proctoeces from Australian teleosts: Variable host-specificity for closely related species identified through multi-locus molecular data. Parasitology International, 66, 16–26.
Xia, X. (2018). DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Molecular Biology and Evolution, 35, 1550–1552.
Xia, X., & Lemey, P. (2009). Assessing substitution saturation with DAMBE. In: Lemey, P., Salemi, M. & Vandamme, A. M. (Eds) Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny. Cambridge: University Press, pp. 615–630.
Xia, X., Xie, Z., Salemi, M., Chen, L. U., & Wang, Y. (2003). An index of substitution saturation and its application. Molecular Phylogenetics and Evolution, 26, 1–7.
Yamaguti, S. (1934). Studies on the helminth fauna of Japan, Part 2. Trematodes of fishes, I. Japanese Journal of Zoology, 5, 249–541.
Yamaguti, S. (1942). Studies on the helminth fauna of Japan. Part 39. Trematodes of fishes mainly from Naha. Transactions of the Biogeographical Society of Japan, 3, 329–398.
Yamaguti, S. (1953). Parasitic worms mainly from Celebes. Part 3. Digenetic trematodes of fishes. II. Acta Medicinae Okayama, 8, 257–295.
Yamaguti, S. (1970). Digenetic trematodes of Hawaiian fishes. Tokyo: Keigaku.
Zhokhov, A. E., Ha, V. T., Oanh, L. T. K., Pugacheva, M. N., & Thanh, N. T. H. (2018). Parasites of anemonefishes (Pomacentridae, Amphiprioninae) in the Gulf of Nha Trang, South China Sea, Vietnam. Zoologicheskii Zhurnal, 97, 1350–1362.
Acknowledgements
We thank members of the Marine Parasitology Laboratory at the University of Queensland for their continued support and assistance in the field. We thank John Page for his assistance in the collection of fish hosts. We also thank staff at the Moreton Bay Research Station for their support. This project represents a contribution to Taxonomy Australia (2020), a national initiative organised under the auspices of the Australian Academy of Science that brings together the taxonomic community to develop approaches that will significantly increase the rate at which new species are discovered, resolved and named, with a view to completely documenting the Australian biota within a generation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by the PADI Foundation (awarded to BD) and the Australian Biological Resources Study (ABRS National Taxonomy Research Grant 4-H04JDSM awarded to SCC and THC).
Author information
Authors and Affiliations
Contributions
All authors collected and prepared the material. B.D. wrote the manuscript and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duong, B., Cribb, T.H. & Cutmore, S.C. Evidence for two morphologically cryptic species of Hysterolecitha Linton, 1910 (Trematoda: Lecithasteridae) infecting overlapping host ranges in Moreton Bay, Australia. Syst Parasitol 100, 363–379 (2023). https://doi.org/10.1007/s11230-023-10092-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10092-6