Abstract
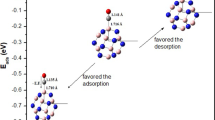
We report adsorption energies, structures, energy gap (E g), charge transfer, and electronic properties of carbon monoxide (CO) on primary, cation Li-, Li-, and two Li-encapsulated fullerene-like beryllium oxide (Be16O16, Li+@Be16O16, Li@Be16O16, and 2Li@Be16O16, respectively) for several adsorption states. The results have been interpreted by DFT calculations. The presented evidence shows that the CO molecule is not strongly adsorbed on the fullerene-like Be16O16 leading to energy release of − 0.17 to − 0.4 eV while its electronic properties did not show significant change. Li+@Be16O16, Li@Be16O16, and 2Li@Be16O16 can adsorb carbon monoxide more strongly than their pristine fullerene-like Be16O16. The energy gap (E g) of the Li@Be16O16 and 2Li@Be16O16 significantly decreased from 3.51 and 2.88 to 2.98 and 2.26 eV, upon the CO adsorption corresponding to the most stable configurations, respectively. It was also shown that the electrical conductance of the Li@Be16O16 and 2Li@Be16O16 may be increased after the CO adsorption. It was found that the electronic properties of Li@Be16O16 and 2Li@Be16O16 are sensitive to the presence of CO molecule.












Similar content being viewed by others
References
Zhuiykov S, Wlodarski W, Li Y (2001). Sensors Actuators B Chem 77:484–490
Fukui K, Nakane M (1995). Sensors Actuators B Chem 25:486–490
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012). Struct Chem 23:653–657
Wanno B, Tabtimsai C (2014). Superlattice Microst 67:110–117
Malavasi L, Tealdi C, Montenero A, Tulliani J, Moggi P, Guglielmi M, Flor G, Lorenzi A, Martucci A, Montanaro L (2006). Sensors Actuators B Chem 118:121–128
Fernández E, Ordejón M, Balbás PLC (2005). Chem Phys Lett 408:252–257
Bludský O, Silhan M, Nachtigall P, Bucko T, Benco L, Hafner J (2005). J Phys Chem B 109:9631–9638
Mino L, Ferrari AM, Lacivita V, Spoto G, Bordiga S, Zecchina A (2011). J Phys Chem C 115:7694–7700
Bechthold P, Pronsato ME, Pistonesi C (2015). Appl Surf Sci 347:291–298
Iijima S (1991). Nature 354:56–58
Peyghan AA, Soleymanabadi, Bagheri HZ (2015). J Iran Chem Soc 12:1071–1076
Ahmadaghaei N, Noei M (2014). J Iran Chem Soc 11:725–731
Murugadoss G, Rajamannan B, Ramasamy V (2011). J Mol Struct 991:202–206
Beheshtian J, Soleymanabadi H, Kamfiroozi M, Ahmadi A (2012). J Mol Model 18:2343–2348
Beheshtian J, Soleymanabadi H, Peyghan AA, Bagheri Z (2013). Appl Surf Sci 268:436–441
Yong Y et al. (2016). Phys Chem Chem Phys 18(31):21431–21441
Yong Y, Lv S, Zhang R, Zhou Q, Su X, Li T, Cui H (2016). RSC Adv 6(92):89080–89088
Yong Y, Lv S, Li X, Li T, Cui H (2015). EPL Europhys Lett 29;111(1):10006
Zhao S, Tian X, Liu J, Ren Y, Ren Y, Wang J (2015). J Clust Sci 26(2):491–503
Huang W, Bulusu S, Pal R, Zeng XC, Wang LS (2009). J Chem Phys 131:234305-1-6
Gao Y, Shao N, Bulusu S, Zeng X (2008). J Phys Chem C 112:8234–8238
Hossain D, Hagelberg F, Pittman CU, Saebo S (2007). J Phys Chem C 111:13864–13871
Joshi K, Jain R, Pandya R, Ahuja B, Sharma B (1999). J Chem Phys 111:163–167
Ren L, Cheng L, Feng Y, Wang X (2012). J Chem Phys 137:014309-1-5
Wu W, Lu P, Zhang Z, Guo W (2011). ACS Appl Mater Interfaces 3:4787–4795
Sahariah MB, Ghosh S (2010). J Appl Phys 107:083520-1-6
Zahedifar M, Mehrabi M, Modares M, Harooni S (2012). J NanoStruct 1:199–203
Xiaofeng W, Richu W, Chaoqun P, Tingting L, Bing L (2011). J Mater Sci Technol 27:147–152
Hwang DY, Mebe AM (2001). Chem Phys Lett 348:303–310
Noei M, Shahabadi VZ, Razi SN (2013). Chin J Chem Phys 26:612–616
Shinde R, Tayade M (2014). J Phys Chem C 118:17200–17204
Samadizadeh M, Rastegar SF, Peyghan AA (2015). Struct Chem 26:809–814
Rastegar SF, Peyghan AA, Soleymanabadi H (2015). Phys E 68:22–27
Dahlke EER, Olson M, Leverentz HR, Truhlar DG (2008). J Phys Chem A 112:3976–3984
Merrick JP, Moran D, Radom L (2007). J Phys Chem A 111:11683–11700
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993). J Comput Chem 14:1347–1363
O'boyle NM, Tenderholt AL, Langner KM (2008). J Comput Chem 29:839–845
Baima J, Erba A, Rérat M, Orlando R, Dovesi R (2013). J Phys Chem C 117:12864–12872
Groh D, Pandey R, Sahariah MB, Amzallag E, Baraille I, Rerat M (2009). J Phys Chem Solids 70:789–795
Li XJ (2009). J Mol Struct THEOCHEM 896:25–29
MatsuoY OH, Maruyama M, Sato H, Tobita H, Ono Y, Omote K, Kawachi K, Kasama Y (2012). Org Lett 14:3784–3787
Ueno H, Nakamura Y, Ikuma N, Kokubo K, Oshima T (2012). Nano Res 5:558–564
Ueno H, Kokubo K, Kwon E, Nakamura Y, Ikuma N, Oshima T (2013). Nano 5:2317–2321
Acknowledgments
Special thanks are due to Dr. Masoumeh Ghalkhani for useful discussions. This work was supported by Shahid Rajaee Teacher Training University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beheshtian, J., Ravaei, I. Toxic CO detection by Li-encapsulated fullerene-like BeO. Struct Chem 29, 231–241 (2018). https://doi.org/10.1007/s11224-017-1022-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-1022-z