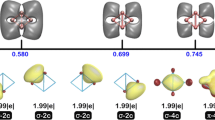
Summary
The nature of the bonding interactions in the recently synthesized first stable neutral complex of so-called zero-valent beryllium (i.e. formally Be(0)) is investigated using the analysis of domain-averaged Fermi holes (DAFHs) and of multicenter bond indices. It is shown that both of these types of analysis, which have previously proved useful for various molecules with nontrivial bonding patterns, basically corroborate the appealing model suggested in the original study to explain the stability of the complex (except for a more realistic specification of the actual valence state of the Be atom). Nevertheless, as well as confirming the anticipated dominance of three-center two-electron π bonding in the central C−Be−C fragment, reinforced by the existence of two donor-acceptor Be−C σ bonds, a more detailed scrutiny of the multicenter bond indices also reveals somewhat unexpected features which suggest also the existence of delocalized 3c-4e σ bonding in the C−Be−C skeleton.





Similar content being viewed by others
Notes
For the sake of mathematical rigor it is also possible to reformulate the whole approach in terms of density matrices instead of densities [45]; this choice of formulation has no practical impact on the actual application of the DAFH analysis to a particular system.
References
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, 2nd edn. Reed Educational and Professional Publishing, Oxford
Herrmann WA, Runte O, Artus G (1995) Synthesis and structure of an ionic beryllium-“carbene” complex. J. Organometal Chem. 501:C1–C4
Arnold T, Braunschweig H, Ewing WC, Kramer T, Mies J, Schuster JK (2015) Beryllium bis(diazabororyl): old neighbors finally shake hands. Chem Commun 51:737–740
Gilliard Jr RJ, Abraham MY, Wang Y, Wei P, Xie Y, Quillian B, Schaefer III HF, Schleyer PR, Robinson G (2012) Carbene-Stabilized Beryllium Borohydride. J Am Chem Soc 134:9953–9955
Arrowsmith M, Hill MS, Kociok-Köhn G, MacDougall DJ, Mahon MF, Mallov I (2012) Three-Coordinate Beryllium β-Diketiniminates: Synthesis and Reduction Chemistry. Inorg Chem 51:13408–13418
Arrowsmith M, Hill MS, Kociok-Köhn G (2015) Activation of N-heterocyclic carbenes by {BeH2} and {Be(H)(Me)} fragments. Organometallics 34:653–662
Dehnicke K, Neumüller B (2008) Neues aus der Chemie des Berylliums. Z Anorg Allg Chem 634:2703–2728
Petz W, Dehnicke K, Holzmann B, Frenking G, Neumüller B (2011) The Reaction of BeCl2 with Carbodiphosphorane C(PPh3)2: Experimental and Theoretical Studies. Z Anorg Allg Chem 637:1702–1710
Azam SS, Hofer TS, Bhattacharjee A, Lim LHV, Pribil AB, Randorf RR, Rode B (2009) Beryllium (II): The Strongest Structure-Forming Ion in Water? A QMCF MD Simulation Study. J Phys Chem B 113:9289–9295
Parameswaran P, Frenking G (2010) Chemical Bonding in Transition Metal Complexes with Beryllium Ligands [(PMe3)2M-BeCl2], [(PMe3)2M-BeClMe], and [(PMe3)2M-BeMe2] (M=Ni,Pd,Pt). J Phys Chem A 114:8529–8535
Li S, Yang X-J, Liu Y, Zhao Y, Li Q-S, Xie Y, Schaefer HF, Wu B (2011) Binuclear Alkaline Earth Metal Compounds (Be,Mg,Ca,Sr,Ba) with α-Diimine Ligands: A Computational Study. Organometallics 30:3113–3118
Couchman SA, Holznann N, Frenking G, Wilson DJD, Dutton JL (2013) Beryllium chemistry the safe way: a theoretical evaluation of low-oxidation state beryllium compounds. Dalton Trans 42:11375–11384
Velasquez A, Fernandez I, Frenking G, Merino G (2007) Multimetallocenes. A Theoretical Study. Organometallics 26:4731–4736
De S, Parameswaran P (2013) Neutral tricoordinated beryllium(0) compounds-isostructural to BH3 but isoelectronic to NH3. Dalton Trans 42:4650–4656
El Khatib M, Bendazzoli GL, Evangelisti S, Helal W, Leininger T, Tenti L, Angeli C (2014) Beryllium Dimer: A Bond Based on Non-Dynamical Correlation. J Phys Chem A 118:6664–6673
Tonner R, Öxler F, Neumüller B, Frenking G (2006) Carbodiphosphoranes: The Chemistry of divalent Carbon C(0). Angew Chem Int Ed 45:8038–8042
Frenking G, Tonner R (2009) Divalent Carbon(0) Compounds. Pure Appl Chem 81:597–614
Tonner R, Frenking G (2008) Divalent Carbon(0) Chemistry, Part 1: Parent Compounds. Chem Eur J 14:3260–3272
Tonner R, Frenking G (2008) Divalent Carbon(0) Chemistry, Part 2: Protonation of Complexes with Main Group and Transition Metal Acids. Chem Eur J 14:3273–3289
Tonner R, Frenking G (2007) C(NHC)2: Divalent Carbon(0) Compounds with N-Heterocyclic Carbene Ligands-Theoretical Evidence for a Class of Molecules with Promising Chemical Properties. Angew Chem Int Ed 46:8695–8698
Ramirez F, Desai NB, Hansen B, McKelvie N (1961) Hexaphenylcarbodiphosphorane (C6H5)3PCP(C6H5)3. J Am Chem Soc 83:3539–3540
Petz W, Kutschera C, Heithaum M, Frenking G, Tonner R, Neumüller B (2008) Experimental and Theoretical Studies Of Carbodiphosphorane-CX2 Adducts With Unusual Bonding Situations: Preparation, Crystal Structures And Bonding Analyses of S2CC(PPh3)2, O2CC(PPh3)2 and [(CO)4MS2CC(PPh3)2] (M = Cr,Mo,W). Inorg Chem 44:1263–1274
Jones ND, Cavell RG (2005) Phosphorus-substituted carbene complexes: chelates, pincers and spirocycles. J Organometal Chem 690:5485–5496
Mondal KC, Roesky HW, Schwarzer MC, Frenking G, Niepster B, Wolf H, Herbst-Irmer R, Stalke D (2013) A Stable Singlet Biradicaloid Siladicarbene: (L:)2Si. Angew Chem Int Ed 52:2963–2967
Xiong Y, Yao S, Inoue S, Epping JD, Driess M (2013) A Cyclic Silylone (“Siladicarbene”) with an Electron-Rich Silicon(0). Angew Chem Int Ed 52:7147–7150
Li Y, Mondal KC, Roesky HW, Zhu H, Stollberg P, Herbst-Irmer R, Stalke D, Andrada DM (2013) Acyclic germylones: Congeners of Allenes with a Central Germanium Atom. J Am Chem Soc 135:12422–12428
Xiong Y, Yao S, Tan G, Inoue S, Driess M (2013) A Cyclic Germadicarbene (“Germylone”) from Germyliumylidene. J Am Chem Soc 135:5004–5007
Kuwabara T, Nakada M, Hamada J, Guo DJ, Nagase S, Saito M (2016) (η4-Butadiene)Sn(0) Complexes: A New Approach For Zero-Valent P-Block Elements Utilizing a Butadiene as a 4π-Electron Donor. J Am Chem Soc 138:11378–11382
Kinjo R, Donnardieu B, Celik MA, Frenking G, Bertrand G (2011) Synthesis and Characterization of a Neutral Tricoordinate Organoboron Isoelectronic with Amines. Science 333:610–613
Kong L, Li Y, Ganguly R, Vidovic D, Kinjo R (2014) Isolating Bs(oxazol-2-ylidene)-Phenylborene Adduct and its Reactivity as a Boron-centered Nucleohile. Angew Chem Int Ed 53:9280–9283
Braunschweig H, Dewhurst RD, Hupp F, Nutz M, Radacki K, Tate CW, Vargas A, Ye Q (2015) Multiple complexation of CO and related ligands to a main-group element. Nature 522:327–330
Arrowsmith M, Braunschweig H, Ali Celik M, Dellermann T, Dewhurst RD, Ewing WC, Hammond K, Kramer T, Krummenacher I, Mies J, Radacki K, Schuster JK (2016) Neutral zero-valent s-block complexes with strong multiple bonding. Nature Chem 8:890–894
Sanigrahi AB, Kar T (1990) Three-center bond index. Chem Phys Lett 173:569–572
Giambiagi M, de Giambiagi MS, Mundim KC (1990) Definition of a Multicenter Bond Index. Struct Chem 1:423–427
Mundim KC, Giambiagi M, de Giambiagi MS (1994) Multicenter Bond Index: Grassmann Algebra and N-Order Density Functional. J Phys Chem 98:6118–6119
Ponec R (1997) Electron Pairing And Chemical Bonds. Chemical Structure, Valences and Structural Similarities from the Analysis of the Fermi holes. J Math Chem 21:323–333
Ponec R (1998) Electron Pairing and Chemical Bonds. Molecular Structure from the analysis of Pair Densities and Related Quantities. J Math Chem 23:85–103
Ponec R, Duben AJ (1999) Electron Pairing and Chemical Bonds. Bonding in Hypervalent Molecules from Analysis of Fermi holes, J. Comp. Chem. 760–771
Ponec R, Roithová J (2001) Domain-averaged Fermi holes – a new means of visualization of chemical bonds. Bonding in hypervalent molecules. Theor Chem Accounts 105:383–392
Ponec R, Yuzhakov G, Cooper DL (2004) Multicenter Bonding and the Structures of Electron-Rich Molecules. Model of Three-Center-Four-Electron Bonding Reconsidered. Theor Chem Accounts 112:419–430
Ponec R, Feixas F (2009) Peculiarities of Multiple Cr-Cr Bonding. Insights from the Analysis of Domain-averaged Fermi Holes. J Phys Chem A 113:8194–8400
Ponec R, Ramos-Cordoba E, Salvador P (2013) Bonding Quandary in the [Cu3S2]3+ Core: Insights from the Analysis of Domain-averaged Fermi Holes and the Local Spin. J Phys Chem A 117:1975–1982
Ponec R (2015) Structure and bonding in binuclear metal carbonyls. Classical paradigms vs. insights from modern theoretical calculations. Comp. Theor. Chem. 1053:195–213
McWeeny R (1960) Some Recent Advances in Density Matrix Theory. Rev Mod Phys 32:335–369
Ponec R, Bučinský L, Gatti C (2010) Relativistic Effects on Metal-Metal Bonding: Comparison of Performance of ECP and Scalar DHK Description on the Picture of Metal-Metal Bonding in Re2Cl8 (2-). J Comp Theory Comput 6:3113–3121
Cioslowski J (1990) Isopycnic Orbital Transformation and Localization of Natural Orbitals. Int J Quant Chem 38(S24):15–28
Bader RFW (1994) Atoms in Molecules. A Quantum Theory. Clarendon Press, Oxford
Ponec R, Cooper DL, Savin A (2008) Analytic Models of Domain-Averaged Fermi Holes. Chem Eur J 14:3338–3345
Cooper DL, Ponec R, Kohout M (2015) New insights from domain-averaged Fermi holes and bond order analysis into the bonding conundrum in C2. Mol Phys 114:1270–1284
Ponec R, Cooper DL (2007) Anatomy of Bond Formation. Domain-Averaged Fermi Holes as a Tool for the Study of the Nature of Chemical Bonding in Li2, Li4 and F2. J Phys Chem A 111:11294–11301
Ponec R, Cooper DL (1997) Anatomy of bond formation. Bond length dependence of the extent of electron sharing in chemical bonds from the analysis of domain-averaged Fermi holes. Faraday Disc 135:31–42
Wiberg KB (1968) Application of Pople-Santry-Segal CNDO method to cyclopropylcarbinyl and cyclobutylcation and to bicyclobutane. Tetrahedron 24:1083–1096
Mayer I (1983) Charge, bond order and valence in the ab initio theory. Chem Phys Lett 97:270–274
Bochicchio R, Ponec R, Torre A, Lain L (2001) Multicenter bonding within the AIM theory. Theor Chem Accounts 105:292–298
Bochicchio R, Lain L, Torre A, Ponec R (2000) Topological population analysis from higher order densities. I. Hartree-Fock level. J Math Chem 28:83–90
Ponec R, Uhlík F (1996) Multicenter Bond Indices from the Generalized Population Analysis of Higher Order Densities. Croat Chem Acta 69:941–954
Bochicchio R, Torre A, Lain L, Ponec R (2000) Topological population analysis from higher order densities. II. The correlated case. J Math Chem 32:241–247
Ponec R, Cooper DL (2004) Generalized Population Analysis of Three-Center Two-Electron Bonding. Int J Quant Chem 97:1002–1011
Feixas F, Rodriguez-Mayorga M, Matito E, Sola M (2015) Three-center bonding analyzed from correlated and uncorrelated third-order reduced density matrices. Comp. Theor. Chem. 1053:173–179
Feixas F, Sola M, Barroso JM, Ugalde JM, Matito E (2014) New Approximation to the Third-Order Density. Application to the Calculation of Correlated Multicenter Indices. J Chem Theor Comp 10:3055–3065
Lain L, Torre A, Bochicchio R (2004) Studies of Population Analysis at the Correlated Level: Determination of Three-Center Bond Indices. J Phys Chem A 108:4132–4137
Francisco E, Pendás ÁM, Garcia-Revilla M, Alvarez-Boto R (2013) A hierarchy of chemical bonding indices in real space from reduced density matrices and cumulants. Comp Theor Chem 1003:71–78
Mandado M, Ponec R (2009) Electron reorganization in allowed and forbidden pericyclic reactions: multicenter bond indices as a measure of aromaticity and/or antiaromaticity in transition states of pericyclic electrocyclizations. J Phys Org Chem 22:1225–1232
Feixas F, Matito E, Poater J, Solà M (2015) Quantifying aromaticity with electron delocalization measures. Chem Soc Rev 44:6434–6451
Mayer I (2012) Improved definition of bond orders for correlated wave functions. Chem Phys Lett 544:83–86
Cioslowski J, Mixon ST (1991) Covalent Bond Orders in the Topological Theory of Atoms in Molecules. J Am Chem Soc 113:4142–4145
Mitoraj MP, Michalak A, Ziegler T (2009) A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J Chem Theory Comp 5:962–975
Michalak M, Mitoraj M, Ziegler T (2008) Bond Orbitals for Chemical Valence Theory. J Phys Chem A 112:1933–1939
Frenking G, Bickelhaupt MF (2014) The EDA Perspective of Chemical Bonding, in The Chemical Bond: Fundamental Aspects of Chemical Bonding (eds G. Frenking and S. Shaik), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, doi: 10.1002/9783527664696.ch4
Pipek J, Mezey PG (1989) A fast intrinsic localization procedure applicable for ab initio and semiempirical linear combination of atomic orbital wave functions. J Chem Phys 90:4916–4926
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, PMW G, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian03, Revision C.02. Gaussian, Inc., Wallingford CT
Werner H-J, Knowles PJ (1985) A 2nd order multiconfiguration SCF procedure with optimum convergence. J Chem Phys 82:5053–5063
Knowles PJ, Werner H-J (1985) An efficient 2nd-order MC SCF method for long configuration expansions. Chem Phys Lett 115:259–267
Knowles PJ, Knowles PJ, Knizia G, Manby FR, Schütz M (2012) Molpro: a general-purpose quantum chemistry program package, WIREs Comput. Mol Sci 2:242–253
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M, Celani P, Györffy W, Kats D, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Shamasundar KR, Adler TB, Amos RD, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Liu Y, Lloyd AW, Mata RA, May AJ, McNicholas, SJ, Meyer W, Mura ME, Nicklass A, O’Neill DP, Palmieri P, Peng D, Pflüger K, Pitzer R, Reiher M, Shiozaki T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M MOLPRO, version 2015.1, a package of ab initio programs: Cardiff, U.K
Keith TA (2012) AIMAll (Version 13.11.04). TK Gristmill Software: Overland Park KS, USA
Parr RG, Ayers PW, Nalewajski RF (2005) What is an atom in a molecule? J Phys Chem A 109:3957–3959
Coulson CA (1961) Valence, 2nd ed., Ch. 8, Oxford University Press Oxford
Moffitt W (1950) Term values in hybrid states. Proc R Soc London, A 202:534–547
Flükiger P, Lüthi HP, Portmann S, Weber J (2000–2002) Molekel: Swiss Center for Scientific Computing
Varetto U (2009) Molekel version 5.4.0.8: Swiss National Supercomputing Center
Zhurko GA ChemCraft version 1.6 (build 294)
Ponec R, Mayer I (1997) Investigation of Some Properties of Multicenter Bond Indices. J Phys Chem A 101:1738–1741
Kar T, Sánchez Marcos E (1992) Three-center four electron bonds and their indices. Chem Phys Lett 192:14–20
Dutton JL, Frenking G (2016) New Avenues in s-Block Chemistry: Beryllium (0) Complexes. Angew Chem Int Ed 55:13380–13382
Acknowledgments
The authors thank Prof. István Mayer (Hungarian Academy of Sciences, Budapest) for stimulating discussions and critical comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponec, R., Cooper, D.L. Insights from domain-averaged Fermi hole (DAFH) analysis and multicenter bond indices into the nature of Be(0) bonding. Struct Chem 28, 1033–1043 (2017). https://doi.org/10.1007/s11224-017-0914-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0914-2