Abstract
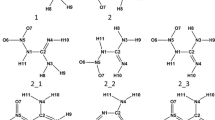
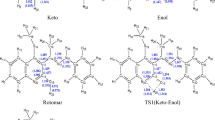
This study focuses on elucidating the stable forms of a new energetic material that is a member of the class of insensitive munitions (IM), 5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO), including its tautomers, and anions. The geometry and properties of all compounds were calculated using density functional theory (M06-2X) and MP2 quantum chemical approaches. Calculations were carried out in the gas phase and in aqueous solution. Chemical stability of these compounds was evaluated in terms of the Gibbs free energy change. Two different solvation models were applied (CPCM and PCM). Calculations showed that overall differences in the results obtained using these two solvation models are negligible for all compounds considered. All possible NTO tautomers were examined and the results are in good agreement with previous studies performed in the gas phase. The stability order was revealed to be slightly dependent on the method applied. In order to estimate acidic properties of NTO, anions of several NTO tautomers were analyzed. In addition, pK a values were calculated using different approaches. As compared with available experimental data it was found that the conductor-like screening model for real solvents approach leads to more accurate estimation of the pK a value than the CPCM and PCM approaches. The pK a value calculated using PCM and CPCM data showed large errors; however, it was proven that the pattern of deprotonation energy was correctly estimated.


Similar content being viewed by others
References
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112(1–2):1–15. doi:10.1016/j.jhazmat.2004.04.003
Sorescu DC, Sutton TRL, Thompson DL, Beardall D, Wight CA (1996) Theoretical and experimental studies of the structure and vibrational spectra of NTO. J Mol Struct 384(2–3):87–99. doi:10.1016/S0022-2860(96)09343-X
Kosowski BMT RC (1996) New processing aid and emulsifier for energetics. Paper presented at the Int Annu Conf ICT (27th)
Le Campion L, Giannotti C, Ouazzani J (1999) Photocatalytic degradation of 5-nitro-1,2,4-Triazol-3-one NTO in aqueous suspention of TiO2. Comparison with fenton oxidation. Chemosphere 38(7):1561–1570. doi:10.1016/S0045-6535(98)00376-2
Cronin MP, Day AI, Wallace L (2007) Electrochemical remediation produces a new high-nitrogen compound from NTO wastewaters. J Hazard Mater 149(2):527–531. doi:10.1016/j.jhazmat.2007.08.007
Wallace L, Cronin MP, Day AI, Buck DP (2009) Electrochemical method applicable to treatment of wastewater from nitrotriazolone production. Environ Sci Technol 43(6):1993–1998
Harris NJ, Lammertsma K (1996) Tautomerism, ionization, and bond dissociations of 5-nitro-2,4-dihydro-3H-1,2,4-triazolone. J Am Chem Soc 118(34):8048–8055
Wang YM, Chen C, Lin ST (1999) Theoretical studies of the NTO unimolecular decomposition. J Mol Struct 460:79–102
Türker L, Atalar T (2006) Quantum chemical study on 5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO) and some of its constitutional isomers. J Hazard Mater 137(3):1333–1344
Spear RJ, Louey CN, Wolfson MG (1989) A preliminary assessment of 3-nitro-1,2,4-triazol-5-one (NTO) as an insensitive high explosive. Material Research Laboratory, Melbourne
Volk F, Bathelt H (1997) Influence of energetic materials on the energy-output of gun propellants. Propellants Explos Pyrotech 22(3):120–124. doi:10.1002/prep.19970220305
Becuwe A, Delclos A (1993) Low-sensitivity explosive compounds for low vulnerability warheads. Propellants Explos Pyrotech 18(1):1–10. doi:10.1002/prep.19930180102
Lee KY, Coburn MD (1985) 3-Nitro-1,2,4-triazol-5-one, a less sensitive explosive explosive, vol LA-10302-MS. Los Alamos National Laboratory, Los Alamos
Lee KY, Coburn MD (1988) 3-Nitro-1,2,4-triazol-5-one, a less sensitive explosive, US Patent No. 4733610
Lee K-Y, Chapman LB, Cobura MD (1987) 3-Nitro-1,2,4-triazol-5-one, a less sensitive explosive. J Energ Mater 5(1):27–33. doi:10.1080/07370658708012347
Chipen GI, Bokalder RP, Grinshtein VY (1966) 1,2,4-Triazol-3-one and its nitro and amino derivatives. Chem Heterocycl Compd 2(1):79–83. doi:10.1007/bf00955602
Langlet A (1990) 3-Nitro-1.2.4-Triazole-5-One (NTO): a new explosive with high performance and low sensitivity. FOA Report C 20789-2
Smith MW, Cliff MD (1999) NTO-based explosive formulations: a technology review. DSTO Aeronautical and Maritime Research Laboratory, Australia
Kröger C-F, Miethchen R, Frank H, Siemerl M, Pilz S (1969) Über 1.2.4-triazole, XVII. Die Nitrierung und Bromierung von 1.2.4-Triazolonen. Chem Ber 102(3):755–766. doi:10.1002/cber.19691020307
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46(7):618–622
Zhao Y, Truhlar D (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1–3):215–241. doi:10.1007/s00214-007-0310-x
Frisch MJ, G. W. T., Schlegel HB, Scuseria GE, Robb MA, J. R. C., Scalmani G, Barone V, Mennucci B, Petersson GA, H. N., Caricato M, Li X, Hratchian HP, Izmaylov AF, J. B., Zheng G, Sonnenberg JL, Hada M, Ehara M, K. T., Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, O. K., Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, F. O., Bearpark M, Heyd JJ, Brothers E, Kudin KN, V. N. S., Keith T, Kobayashi R, Normand J, Raghavachari K, A. R., Burant JC, Iyengar SS, Tomasi J, Cossi M, N. R., Millam JM, Klene M, Knox JE, Cross JB, Bakken V, C. A., Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, A. J. A., Cammi R, Pomelli C, Ochterski JW, Martin RL, K. M., Zakrzewski VG, Voth GA, Salvador P, J. J. D., Dapprich S, Daniels AD, Farkas O, J. B. F., Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. Vol Revision C.01, Gaussian Inc, Wallingford
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255(4–6):327–335. doi:10.1016/0009-2614(96)00349-1
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24(6):669–681
Zhan CG, Dixon DA (2001) Absolute hydration free energy of the proton from first-principles electronic structure calculations. J Phys Chem A 105(51):11534–11540
Tissandier MD, Cowen KA, Feng WY, Gundlach E, Cohen MH, Earhart AD, Coe JV, Tuttle TR (1998) The proton’s absolute aqueous enthalpy and gibbs free energy of solvation from cluster-ion solvation data. J Phy Chem A 102(40):7787–7794. doi:10.1021/jp982638r
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99(7):2224–2235
Klamt A, Eckert F (2000) COSMO-RS: a novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib 172(1):43–72. doi:10.1016/s0378-3812(00)00357-5
Kholod YA, Muratov EN, Gorb LG, Hill FC, Artemenko AG, Kuz’min VE, Qasim M, Leszczynski J (2009) Application of quantum chemical approximations to environmental problems: prediction of water solubility for nitro compounds. Environ Sci Technol 43(24):9208–9215. doi:10.1021/es902566b
HyperChem(TM), Hypercube, Inc., Gainesville, FL
Bell RP (1973) Proton in Chemistry, 2nd edn. Chapman and Hall, London
Acknowledgments
This work was facilitated by support from the High Performance Computing Distributed Shared Resource Center (DSRC) at the ERDC (Vicksburg, MS). The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the U.S. Government. Results in this study were funded and obtained from research conducted under the Environmental Quality Technology Program of the United States Army Corps of Engineers by the US Army ERDC. Permission was granted by the Chief of Engineers to publish this information. The findings of this report are not to be construed as an official Department of the Army position unless so designated by other authorized documents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Golius, A., Gorb, L., Michalkova Scott, A. et al. Computational study of NTO (5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one) tautomeric properties in aqueous solution. Struct Chem 26, 1281–1286 (2015). https://doi.org/10.1007/s11224-014-0526-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0526-z