Abstract
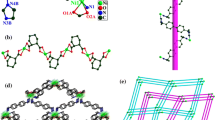
Two energetic catalysts, 3,5-dinitro-2-pyridonate of lead (II) (Pb(2DNPO)2, 1) and 3,5-dinitro-4-pyridone-N-hydroxylate tetrahydrate of copper (II) (Cu(4DNPOH)2(H2O)4, 2) were characterized by elemental analysis, FT-IR, TG-DSC and structurally characterized by X-ray single-crystal diffraction analysis. X-ray powder diffraction analysis of complex 1 confirmed the phase homogeneity of the polycrystalline sample. Crystal data for 1: monoclinic, space group P 21/n, a = 8.5253(9), b = 9.2938(10), c = 19.654(2) Å, β = 102.289(2)°, V = 1521.6(3) Å3, Z = 4; 2: monoclinic, space group P 21/n , a = 8.3705(10), b = 9.9307(12), c = 10.5771(12) Å, β = 98.021(2)°, V = 870.62(18) Å3, Z = 2. The complex 1 is a one-dimensional coordination polymer with each Pb(II) atom being six-coordinated, forming a heavily distorted octahedral geometry, by two nitrogen and four oxygen donors. Each ligand links the Pb(II) ions in a chelating bridging mode using nitrogen and oxygen atoms of its pyridonate part. The complex 2 is a copper (II) complex with a compressed octahedral geometry. The Cu(II) atom locates on the equatorial positions defined by oxygen atoms of four water molecules. Its axial positions are filled with two oxygen donors of the pyridine-N-hydroxylate moieties of two ligands. The abundant hydrogen bonds link the molecules into one-dimensional chains. Both the complexes represent the first two examples of the energetic catalysts containing dinitropyridine derivatives characterized crystallographically







Similar content being viewed by others
References
(a) Denisyuk1 AP, Demidova1 L (2004) Combust Explos Shock Waves 40:311 (b) Hewkin DJ, Hicks JA, Powling J, Watts SH (1971) Combust Sci Technol 2:307 (c) Denisyuk AP, Demidova LA, Galkin VI (1995) Combust Explos Shock Waves 31:61
(a) Pundlik SM, Palaiah, RS, Nair JK et al (2001) J Energ Mater 19:339 (b) Klapotke TM, Rienacker CM (2001) Propellants Explos Pyrotechnics 26:43
Patchornik A (2002) Polym Adv Technol 13:1078
(a) Kaye SM (1978) Encyclopedia of Explosives and Related Items, U.S. Army ARDEC, Dover, NJ 8:274 (b) Brill TB, Brush PJ, James KJ, Shepherd JE, Pfeiffer KJ (1991) Appl Spectrosc 46:900
(a) Meng ZH, Hu RZ, Therm Anal (1995) 45:79 (b) Brill TB, Zhang TL, Tappan BC (2000) Combust Flame 121:662 (c) Wang YM, Chen C, Lin ST (1999) J Mol Struct (Theochem) 460:79
Coburn MD, Lee KY (1990) J Heterocycl Chem 27:575
(a)Song JR, Hu RZ, Kang B, Li FP (1999) Thermochim Acta 331:49 (b) Song JR, Chen ZX, Xiao HM, Hu RZ, Li FP (1999) Chin Sci Bull 44:214
Yang YM, Zhang TL, Zhang JG, Shao B, Yu KB (2002) Chin J Struct Chem 21:321
Fang GY, Xu LN, Xiao HM, Ju XH (2005) Acta Chemica Sinica 63:1055
Zhang TL, Wang SZ, Yang L, Sun YH, Zhang JG, Qiao XJ (2006) Chin J Inorg Chem 22:1819
Zhao FQ, Chen P, Luo Y, Zhang RE, Li SW, Deng MZ, Zhen YM (2003) Chin J Explos Propellants 3:1
Chen P, Zhao FQ, Luo Y, Hu RZ, Zheng YM, Deng MZ, Gao Y (2004) Acta Chemica Sinica 13:1197
Zhen YM, Deng MZ, Zhao FQ, Yuan C (2002) Chin J Explos Propellants 4:51
Tanogaki M, Komura S (1994) JP 06220019
Twieg R, Azema A, Jain K, Cheng YY (1982) Chem Phys Lett 92:208
Driscoll PR, Fords NJ (1970) US Patent no. 3495969
(a) Kemmitt T, Hubert-Pfalzgraf LG, Gainsford GJ, Richard P (2005) Inorg Chem Commun 8:1149 (b) Vassilyeva OY, Kokozay VN, Zhukova NA, Kovbasyuk LA (1997) Polyhedron 16:263
(a) Liat SL, Jenny PG, Charles WB (1998) Inorg Chem 37:1853 (b) Cecconi F, Ghilardi CA, Midollini S, Orlandini A (2000) Inorganica Chimica Acta 308:135
Mahjoub AR, Morsali A (2002) Polyhedron 21:1223
Chen P, Zhao FQ, Luo Y, Hu RZ, Gao SL, Zheng YM, Deng MZ, Gao Y (2004) Chin J Chem 22:1056
Acknowledgments
We thank the Postgraduate-Innovation-Foundation of Shaanxi Normal University and the Combustion Key Laboratory of Xi’an Modern Chemistry Research Institute for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
She, JB., Zhang, GF., Zhao, FQ. et al. The first two structurally characterized energetic catalysts derived from dinitropyridone. Struct Chem 18, 373–378 (2007). https://doi.org/10.1007/s11224-007-9179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-007-9179-5