Abstract
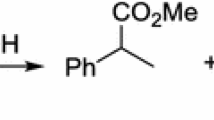
The complexes (dpp-bian)Mg(thf)3, (dpp-bian)Ca(thf)4 and (dpp-bian)Mg(pyr)3 (dpp-bian is the 1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene dianion; pyr is the pyrrolidine) catalyze the addition of pyrrolidine to 2-vinylpyridine at room temperature. The compound (dppbian)Mg[N(SiMe3)2] containing a dpp-bian radical anion catalyzes the addition of pyrrolidine to styrene at 60 °C. The dpp-bian radical anion lithium-sodium salt [(dpp-bian)Li{N-(SiMe3)2}][Na(C7H8)] is an active catalyst of the addition of pyrrolidine to styrene and isoprene at 60 °C. In all the case, the content of the catalyst was from 1 to 2 mol.%. For styrene and 2-vinylpyridine, the reactions proceeded with the formation of anti-Markovnikov addition product, while 1,4-addition product was obtained in the case of isoprene.
Similar content being viewed by others
References
L. Huang, M. Arndt, K. Gooßen, H. Heydt, L. J. Gooßen, Chem. Rev., 2015, 115, 2596.
N. T. Patil, R. D. Kavthe, V. S. Shinde, Tetrahedron, 2012, 68, 8079.
A. Corma, A. Leyva-Pérez, M. J. Sabater, Chem. Rev., 2011, 111, 1657.
T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev., 2008, 108, 3795.
J. Hannedouche, E. Schulz, Chem. Eur. J., 2013, 19, 4972.
P. Yin, T. P. Loh, Org. Lett., 2009, 11, 3791.
I. V. Basalov, S. C. Roca, D. M. Lyubov, A. N. Selikhov, G. K. Fukin, Y. Sarazin, J.-F. Carpentier, A. A. Trifonov, Inorg. Chem., 2014, 53, 1654.
S. Germain, E. Schulz, J. Hannedouche, ChemCatChem, 2014, 6, 2065.
A. A. Kissel, T. V. Mahrova, D. M. Lyubov, A. V. Cherkasov, G. K. Fukin, A. A. Trifonov, I. del Rosal, L. Maron, Dalton Trans., 2015, 44, 12137.
J.-S. Ryu, G. Y. Li, T. J. Marks, J. Am. Chem. Soc., 2003, 125, 12584.
D. V. Gribkov, K. C. Hultzsch, F. Hampel, J. Am. Chem. Soc., 2006, 128, 3748.
A. L. Reznichenko, H. N. Nguyen, K. C. Hultzsch, Angew. Chem., Int. Ed., 2010, 49, 8984.
A. L. Reznichenko, K. C. Hultzsch, Organometallics, 2013, 32, 1394.
B. Liu, T. Roisnel, J.-F. Carpentier, Y. Sarazin, Chem. Eur. J., 2013, 19, 13445.
G. M. Barrett, C. Brinkmann, M. R. Crimmin, M. S. Hill, P. Hunt, P. A. Procopiou, J. Am. Chem. Soc., 2009, 131, 12906.
C. Brinkmann, A. G. M. Barrett, M. S. Hill, P. A. Procopiou, J. Am. Chem. Soc., 2012, 134, 2193.
B. Liu, T. Roisnel, J.-F. Carpentier, Y. Sarazin, Angew. Chem., Int. Ed., 2012, 51, 4943.
X. Zhang, T. J. Emge, K. C. Hultzsch, Angew. Chem., Int. Ed., 2012, 51, 394.
M. S. Hill, D. J. Liptrot, C. Weetman, Chem. Soc. Rev., 2016, 45, 972.
Y. Sarazin, J.-F. Carpentier, Chem. Rec., 2016, doi:10.1002/tcr.201600067.
P. Horrillo-Martínez, K. C. Hultzsch, A. Gil, V. Branchadell, Eur. J. Org. Chem., 2007, 3311.
M. Beller, C. Breindl, Tetrahedron, 1998, 54, 6359.
I. L. Fedushkin, A. S. Nikipelov, A. G. Morozov, A. A. Skatova, A. V. Cherkasov, G. A. Abakumov, Chem. Eur. J., 2012, 18, 255.
I. L. Fedushkin, O. V. Kazarina, A. N. Lukoyanov, A. A. Skatova, N. L. Bazyakina, A. V. Cherkasov, E. Palamidis, Organometallics, 2015, 34, 1498.
O. V. Kazarina, M. V. Moskalev, I. L. Fedushkin, Russ. Chem. Bull. (Int. Ed.), 2015, 64, 32 [Izv. Akad. Nauk, Ser. Khim., 2015, 32].
I. L. Fedushkin, M. V. Moskalev, E. V. Baranov, G. A. Abakumov, J. Organomet. Chem., 2013, 747, 235.
M. V. Moskalev, A. M. Yakub, A. G. Morozov, E. V. Baranov, O. V. Kazarina, I. L. Fedushkin, Eur. J. Org. Chem., 2015, 5781.
I. L. Fedushkin, A. S. Nikipelov, K. A. Lyssenko, J. Am. Chem. Soc., 2010, 132, 7874.
I. L. Fedushkin, M. V. Moskalev, A. N. Lukoyanov, A. N. Tishkina, E. V. Baranov, G. A. Abakumov, Chem. Eur. J., 2012, 18, 11264.
I. L. Fedushkin, N. M. Khvoinova, A. A. Skatova, G. K. Fukin, Angew. Chem., Int. Ed., 2003, 42, 5223.
I. L. Fedushkin, Proc. International Conference on Organometallic and Coordination Chemistry (N. Novgorod, September 2–8, 2008), N. Novgorod,2010, S7.
I. L. Fedushkin, A. A. Skatova, V. A. Chudakova, G. K. Fukin, S. Dechert, H. Schumann, Eur. J. Inorg. Chem., 2003, 3336.
I. L. Fedushkin, A. G. Morozov, V. A. Chudakova, G. K. Fukin, V. K. Cherkasov, Eur. J. Inorg. Chem., 2009, 4995.
I. L. Fedushkin, V. A. Chudakova, G. K. Fukin, S. Dechert, M. Hummert, H. Schumann, Russ. Chem. Bull. (Int. Ed.), 2004, 53, 2744 [Izv. Akad. Nauk, Ser. Khim., 2004, 2641].
H. Schumann, M. Hummert, A. N. Lukoyanov, I. L. Fedushkin, Chem. Eur. J. 2007, 13, 4216.
I. L. Fedushkin, A. N. Lukoyanov, M. Hummert, H. Schumann, Russ. Chem. Bull. (Int. Ed.), 2007, 56, 1765 [Izv. Akad. Nauk, Ser. Khim., 2007, 1702].
M. Beller, H. Trauthwein, M. Eichberger, C. Breindl, T. E. Müller, Eur. J. Inorg. Chem., 1999, 1121.
Bruker (2012). APEX2. BrukerAXS Inc., Madison, Wisconsin, USA.
Bruker (2001). SADABS. BrukerAXS Inc., Madison, Wisconsin, USA.
Agilent (2014). CrysAlis PRO. Agilent Technologies Ltd, Yarnton, Oxfordshire, England.
G. M. Sheldrick, ActaCryst., 2015, A71, 3.
G. M. Sheldrick, ActaCryst., 2015, C71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences R. Z. Sagdeev on the occasion of his 75th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 12, pp. 2887–2894, December, 2016.
Rights and permissions
About this article
Cite this article
Yakub, A.M., Moskalev, M.V., Bazyakina, N.L. et al. Hydroamination of 2-vinylpyridine, styrene, and isoprene with pyrrolidine catalyzed by alkali and alkaline-earth metal complexes. Russ Chem Bull 65, 2887–2894 (2016). https://doi.org/10.1007/s11172-016-1673-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1673-8