Abstract
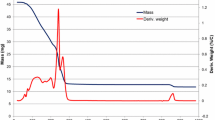
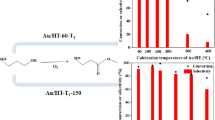
To obtain highly active solid base catalyst of magnesium oxide (MgO) under atmospheric conditions, hydroxide (Mg(OH)2), basic carbonate (Mg5(CO3)4(OH)2·4H2O), and oxalate (MgC2O4·2H2O) were examined as starting material for preparation, and the effect of heating temperature on base catalytic activity was studied. Diacetone alcohol decomposition, a retro-aldol reaction, was performed to compare the base catalytic activity of prepared MgO catalysts. The order of activity of MgO as starting material for the retro-aldol reaction was oxalate > basic carbonate > hydroxide. In a comparison of catalytic activity based on catalyst weight and surface area, MgO prepared from oxalate showed higher activity than did samples prepared from carbonate and hydroxide. Catalytic activity based on surface area was the highest in MgO prepared by thermal decomposition of oxalate at 1173 K. The starting material, which had higher decomposition temperature and formed a larger amount of evolving gas molecules accompanying decomposition, provided a solid base with higher activity under atmospheric conditions.







Similar content being viewed by others
References
A. Corma, S. Iborra, Adv. Catal. 49, 239 (2006)
H. Hattori, Appl. Catal. A: General 222, 247 (2001)
H. Hattori, J. Jpn. Petrol. Inst. 47, 67 (2004)
G. Busca, Chem. Rev. 110, 2217 (2010)
X. Yang, B. Lv, T. Lu, Y. Sua, L. Zhou, Catal. Sci. Technol. 10, 700 (2020)
G.A.H. Mekhemer, S.A. Halawy, M.A. Mohamed, M.I. Zaki, J. Phys. Chem. B 108, 13379 (2004)
J. Li, Y. Le, W.-L. Dai, H. Li, K. Fan, Catal. Commun. 9, 1334 (2008)
J.K. Bartley, C. Xu, R. Lloyd, D.I. Enache, D.W. Knight, G.J. Hutchings, Appl. Catal. B: Environmental 128, 31 (2012)
J. Puriwat, W. Chaitree, K. Suriya, S. Dokjampa, P. Praserthdam, J. Panpranot, Catal. Commun. 12, 80 (2010)
M. Kitagawa, S. Misu, J. Ichikawa, H. Matsuhashi, Res. Chem. Intermed. 41, 9463 (2015)
K.J. Klabunde, H. Matsuhashi, J. Am. Chem. Soc. 109, 1111 (1987)
G.L. Davis, G.H. Burrows, J. Am. Chem. Soc. 58, 311 (1936)
Y. Sawada, J. Yamaguchi, O. Sakurai, K. Uematsu, N. Mizutani, M. Kato, Thermochim. Acta 33, 127 (1979)
K. Nagase, K. Sato, N. Tanaka, Bull. Chem. Soc. Jpn 48, 439 (1975)
R.J. Cvetanovic, Y. Amenomiya, Adv. Catal. 17, 103 (1967)
M.H. Al-Hazmi, Y.-M. Choi, A.W. Apblett, Catal. Lett. 143, 705 (2013)
H. Matsuhashi, D. Funaki, J. Jpn. Petrol. Inst. 59, 24 (2016)
O. Kikhtyanin, V. Kelbichová, D. Vitvarová, M. Kubů, D. Kubička, Catal. Today 227, 154 (2014)
J.F. Goodman, Proc. Roy. Soc. A 247, 346 (1958)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kitagawa, M., Ishida, N., Yoshino, E. et al. Comparison of the base catalytic activity of MgO prepared by thermal decomposition of hydroxide, basic carbonate, and oxalate under atmospheric conditions. Res Chem Intermed 46, 3703–3715 (2020). https://doi.org/10.1007/s11164-020-04169-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04169-w