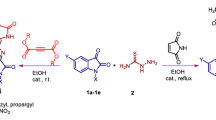
Abstract
We performed a series of novel benzamide compounds which were synthesized starting from 2,3-dimethoxybenzoic acid or 3-acetoxy-2-methylbenzoic acid and amine derivatives. All the obtained products were purified, and the analysis of these products was determined with IR, 1H NMR, 13C NMR spectroscopic, and elemental methods. The in vitro antioxidant activity of all the compounds was determined by total antioxidant, free radical scavenging, and metal chelating activity. Some of synthesized compounds showed more effective total antioxidant, free radical scavenging, and metal chelating activity compared with standards. One of the benzamide compounds has been shown to exhibit effective metal chelate activity. The new compounds were determined in vitro antibacterial activity against three gram-positive bacteria and three gram-negative bacteria and compared with two control drugs. Thus, by conducting in vivo biochemical tests of effective amides, researches can be carried out in different fields of application.
Graphic abstract
We performed a series of novel benzamide compounds which were synthesized, and the analysis of these products was determined with IR, 1H NMR, 13C NMR spectroscopic, and elemental methods. The in vitro antioxidant activity of all the compounds was determined by total antioxidant, free radical scavenging, and metal chelating activity. All the compounds were tested for their in vitro growth inhibitory activity against different bacteria.










Similar content being viewed by others
References
E.A. Şener, K.K. Bingöl, İ. Ören, Ö.T. Arpacı, İ. Yalçın, N. Altanlar, Il Farmaco 55, 6 (2000)
J. Fu, K. Cheng, Z.M. Zhang, R.Q. Fang, H.L. Zhu, Eur. J. Med. Chem. 45, 6 (2010)
N. Kanışkan, Ş. Kökten, İ. Çelik, Arkivoc 8, viii (2012)
R. Tang, L. Jin, C. Mou, J. Yin, S. Bai, D. Hu, J. Wu, S. Yang, B. Song, Chem. Cent. J. 7, 30 (2013)
Y.F. Xiang, C.W. Qian, G.W. Xing, J. Hao, M. Xia, Y.F. Wang, Bioorg. Med. Chem. Lett. 22, 14 (2012)
F. Bettelheim, J. March, Introduction to General, Organic and Biochemistry (Sounders College Publishing, Fort Worth, 1998), p. 492
M. Bhat, S. Belagali, P.R. Shastry, V.R. Rai, Monatsh. Chem. 147, 11 (2016)
D. Carbonnelle, F. Ebstein, C. Rabu, J.Y. Petit, M. Gregoire, F. Lang, Eur. J. Immunol. 35, 2 (2005)
N. Kushwaha, R.K. Saini, S.K. Kushwaha, Int. J. Chem. Tech. Res 3, 203 (2011)
X. Li, Z. Li, H. Deng, X. Zhou, Tetrahedron Lett. 54, 18 (2013)
A.C. Shekhar, A.R. Kumar, G. Sathaiah, V.L. Paul, M. Sridhar, P.S. Rao, Tetrahedron Lett. 50, 50 (2009)
K. Suzuki, H. Nagasawa, Y. Uto, Y. Sugimoto, K. Noguchi, M. Wakida, K. Wierzba, T. Terada, T. Asao, Y. Yamada, Bioorg. Med. Chem. 13, 12 (2005)
B. Yu, Y.L. Li, S.H. Song, X.L. Ji, M.S. Lin, C.F. Wu, Bioorg. Med. Chem. Lett. 22, 1 (2012)
S.N. Karanth, N. Badiadka, S.B. Kunhanna, K.S. Shashidhara, P.P. Yegneswaran, Res. Chem. Intermed. 44, 7 (2018)
K. Rehse, J. Kotthaus, L. Khadembashi, Arch. Pharm. 342, 1 (2009)
A. Graul, J. Castaner, Drugs Future 22, 9 (1997)
J.M. Humphrey, A.R. Chamberlin, Chem. Rev. 97, 6 (1997)
Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu, X. Wan, Angew. Chem. Int. Ed. 51, 3231 (2012)
S. Shankerrao, Y.D. Bodke, S.S. Mety, Med. Chem. Res. 22(3), 1163 (2013)
K. Krumova, G. Cosa, Singlet Oxygen: Applications in Biosciences and Nanosciences, ed. by S. Nonell, C. Flors, vol. 1, chapter 1 (Royal Society of Chemistry, Cambridge, 2016), p. 1
M.E. Jung, S. Abrecht, J. Organ. Chem. 53, 2 (1988)
M. Mazik, D. Bläser, R. Boese, Tetrahedron 55, 44 (1999)
P. Prieto, M. Pineda, M. Aguilar, Anal. Biochem. 269, 2 (1999)
M.S. Blois, Nature 181, 4617 (1958)
T. Özen, M. Taş, J. Enzyme Inhib. Med. Chem. 24, 5 (2009)
M. Sahin, N. Erkan, E. Ayranci, J. Solut. Chem. 45, 1 (2016)
T.C. Dinis, V.M. Madeira, L.M. Almeida, Arch. Biochem. Biophys. 315, 1 (1994)
J.M. Andrews, J. Antimicrob. Chemother. 48(suppl_1), 5 (2001)
Y. Ishii, M. Takeno, Y. Kawasaki, A. Muromachi, Y. Nishiyama, S. Sakaguchi, J. Organ. Chem. 61, 9 (1996)
Y. Watanabe, T. Mukaiyama, Chem. Lett. 10, 3 (1981)
T. Hoegberg, P. Stroem, M. Ebner, S. Raemsby, J. Organ. Chem. 52, 10 (1987)
A.G. Barrett, J.C.A. Lana, J. Chem. Soc. Chem. Commun. 11, 471 (1978)
K. Dooleweerdt, B.P. Fors, S.L. Buchwald, Organ. Lett. 12, 10 (2010)
E. Racine, F. Monnier, J.-P. Vors, M. Taillefer, Organ. Lett. 13, 11 (2011)
K. Phukan, M. Ganguly, N. Devi, Synth. Commun. 39, 15 (2009)
J.B. Feng, D. Wei, J.L. Gong, X. Qi, X.F. Wu, Tetrahedron Lett. 55, 36 (2014)
S. Cakmak, H. Kutuk, M. Odabasoglu, H. Yakan, O. Buyukgungor, Lett. Organ. Chem. 13, 3 (2016)
S. Demir, S. Cakmak, N. Dege, H. Kutuk, M. Odabasoglu, R.A. Kepekci, J. Mol. Struct. 1100, 582 (2015)
A.G. Iriarte, M.F. Erben, K. Gholivand, J.L. Jios, S.E. Ulic, C.O. Della Védova, J. Mol. Struct. 886, 1–3 (2008)
B.K. Kırca, Ş. Çakmak, H. Kütük, M. Odabaşoğlu, O. Büyükgüngör, J. Mol. Struct. 1151, 191 (2018)
J. Liang, B. Liu, Bioeng. Transl. Med. 1, 3 (2016)
M. Cindrić, I. Sović, I. Martin-Kleiner, M. Kralj, T. Mašek, M. Hranjec, K. Starčević. Med. Chem. Res. 26, 9 (2017)
R. Walia, M. Hedaitullah, S.F. Naaz, K. Iqbal, H. Lamba, Int. J. Res. Pharm. Chem. 1, 3 (2011)
S.S. Panga, R. Tamatam, P. Adivireddy, P. Venkatapuram, S.K. Narra, K. Paturu, Res. Chem. Intermed. 45, 5 (2019)
D. Gandhi, P. Kalal, S. Agarwal, Chem. Biol. Interface 7, 2 (2017)
N. Gunasekaran, V. Vadivel, N.R. Halcovitch, E.R. Tiekink, Chem. Data Collect. 9, 263 (2017)
M.A. Demirci, Y. Ipek, F. Gul, T. Ozen, I. Demirtas, Food Chem. 269, 111 (2018)
G.O. Puntel, P. Gubert, G.L. Peres, L. Bresolin, J.B.T. Rocha, M.E. Pereira, V.S. Carratu, F.A.A. Soares, Arch. Toxicol. 82, 10 (2008)
F. Haber, J. Weiss, Naturwissenschaften 20, 51 (1932)
İ.Y. Ören, E.A. Şener, C. Ertaş, Ö.T. Arpaci, İ. Yalçin, N. Altanlar, Turk. J. Chem. 28, 4 (2004)
D. Rai, R.K. Singh, Indian J. Chem. 50B, 7 (2011)
Acknowledgements
This study was supported by Ondokuz Mayıs University as a Scientific Research Project (PYO.FEN.1904.18.018). We would like to thank Dr. Mohammad Maher Jesry for checking and proofreading the article. Moreover, we would like to thank research assistant Yavuz Derin for taking the NMR spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yakan, H., Cakmak, S., Kutuk, H. et al. Synthesis, characterization, antioxidant, and antibacterial activities of new 2,3-dimethoxy and 3-acetoxy-2-methyl benzamides. Res Chem Intermed 46, 2767–2787 (2020). https://doi.org/10.1007/s11164-020-04118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04118-7