Abstract
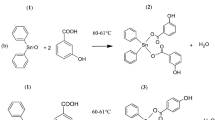
Searching for new active molecules against human breast cancer cell line MCF-7, novel quinoline based thiazolidinones has been efficiently synthesized under ultrasound irradiation. The newly synthesized compounds were tested against human breast cancer cell line MCF-7. Compounds P3, P4, and P6 were found to be promising inhibitors of MCF-7 characterized by lower IC50 values in a dose-dependent mode with high specificity against MCF-7 (IC50 of 10 μM at 24 h). Among all the synthesized compounds, P3, P4, and P6 shows IC50 values 5.38, 5.12, and 0.73 µM, respectively, were considered as a potential lead. These lead molecules showed significant anti-cancer activity against human breast cancer cell line MCF-7. Additionally, induction of G2/M cell arrest within 24 h was discovered via flow cytometry analysis. Overall, our data suggest that potent compounds have an inhibitory effect on cell proliferation of MCF-7 through cell cycle arrest, giving it great potential as a future therapeutic reagent for cancers.




Similar content being viewed by others
References
Cancer incidence and mortality worldwide, Globocan, IARC (2012, 2013). http://globocan.iarc.fr
D. Carta, R. Bortolozzi, E. Hamel, G. Basso, S. Moro, G. Viola, M.G. Ferlin, J. Med. Chem. 58, 7991 (2015)
S. Wilhelm, C. Carter, M. Lynch, T. Lowinger, J. Dumas, R.A. Smith, B. Schwartz, R. Simantov, S. Kelley, Nat. Rev. Drug Discov. 5, 835 (2006)
S.A. Jadhav, A.P. Sarkate, A.V. Raut, D.B. Shinde, Res. Chem. Intermed. 43, 4531 (2017)
S.A.H. El-Feky, Z.K.A. El-Samii, N.A. Osman, J. Lashine, M.A. Kamel, H.K. Thabet, Bioorg. Chem. 58, 104 (2015)
S. Sahu, S.K. Ghosh, J. Kalita, M. Dutta, H.R. Bhat, Exp. Parasitol. 163, 38 (2016)
I. Briguglio, R. Loddo, E. Laurini, M. Fermeglia, S. Piras, P. Corona, P. Giunchedi, E. Gavini, G. Sanna, G. Giliberti, C. Ibba, P. Farci, P.L. Colla, S. Pricl, A. Carta, Eur. J. Med. Chem. 105, 63 (2015)
S. Eswaran, A.V. Adhikari, N.S. Shetty, Eur. J. Med. Chem. 44, 4637 (2009)
V.S. Dofe, A.P. Sarkate, R. Azad, C.H. Gill, Res. Chem. Intermed. (2017). doi:10.1007/s11164-017-3078-1
B. Medapi, J. Renuka, S. Saxena, J.P. Sridevi, R. Medishetti, P. Kulkarni, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. 23, 2062 (2015)
K. Majerz-Maniecka, R. Musiol, A. Skórska-Stania, D. Tabak, P. Mazur, B.J. Oleksyn, J. Polanski, Bioorg. Med. Chem. 19, 1606 (2011)
A. Verma, S.K. Saraf, Eur. J. Med. Chem. 43, 897 (2008)
D.P. Bhoot, R.C. Khunt, V.K. Shankhavara, H.H. Parekh, J. Sci. Islam. Repub. Iran 17, 323 (2006)
G. Cihan-Üstündag, E. Gürsoy, L. Naesens, N. Ulusoy-Güzeldemirci, G. Çapan, Bioorg. Med. Chem. 24, 240 (2016)
M.R. Bhosle, J.R. Mali, S. Pal, A.K. Srivastava, R.A. Mane, Bioorg. Med. Chem. Lett. 24, 2651 (2014)
N.C. Desai, H.V. Vaghani, P.N. Shihora, J. Fluor. Chem. 153, 39 (2013)
Y. Wan, S. Wu, G. Xiao, T. Liu, X. Hou, C. Chen, P. Guan, X. Yang, H. Fang, Bioorg. Med. Chem. 23, 1994 (2015)
K. Arya, D.S. Rawat, A. Dandia, H. Sasai, J. Fluor. Chem. 137, 117 (2012)
K.R.A. Abdellatif, M.A. Abdelgawad, H.A.H. Elshemy, S.S.R. Alsayed, Bioorg. Chem. 64, 1 (2016)
F.A.R. Ruiz, R.N. García-Sánchez, S.V. Estupiñan, A. Gómez-Barrio, D.F.T. Amado, B.M. Pérez-Solórzano, J.J. Nogal-Ruiz, A.R. Martínez-Fernández, V.V. Kouznetsov, Bioorg. Med. Chem. 19, 4562 (2011)
H. Chen, Z. Guo, Q. Yin, X. Duan, Y. Gu, X. Li, Front. Chem. Sci. Eng. 5, 231 (2011)
K.M. Amin, D.E. Rahman, Y.A. Al-Eryani, Bioorg. Med. Chem. 16, 5377 (2008)
T.J. Mason, Chem. Soc. Rev. 26, 443 (1997)
K.A. Metwally, L.M. Abdel-Aziz, E.M. Lashine, M.I. Husseiny, R.H. Badawy, Bioorg. Med. Chem. 14, 8675 (2006)
G.K. Schwartz, M.A. Shah, J. Clin. Oncol. 23, 9408 (2005)
Acknowledgements
Author V.S.D. is very much grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing Senior Research Fellowship and Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh for providing spectral data. We are thankful to Head, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, Maharashtra, for providing laboratory facility. We are thankful to Dean, School of Life Sciences, University of Hyderabad, for providing the FACS facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dofe, V.S., Sarkate, A.P., Azad, R. et al. Green synthesis and inhibitory effect of novel quinoline based thiazolidinones on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Res Chem Intermed 44, 1149–1160 (2018). https://doi.org/10.1007/s11164-017-3157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3157-3