Abstract
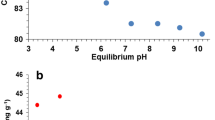
In this paper, the kinetics and equilibrium of removing nickel from galvanic sludge leachate on nanocrystalline (14.6 nm) titania (TiO2) powders were studied for the first time. The pseudo-first-order, Natarajan and Khalaf, Bhattacharya and Venkobachar, the pseudo-second-order, Bangham’s equation, Elovich, and intraparticle diffusion kinetic models were applied in order to identify potential adsorption process mechanisms. The adsorption kinetic data were best represented by the second-order model (k 2 = 0.041 × 102 g mg−1 min−1). Thermodynamic parameters were also calculated to study the effect of temperature on the removal process. The positive Gibbs free energy (∆G°) and positive enthalpy change (∆H°) indicated the non-spontaneous and endothermic nature of the reaction, respectively. The equilibrium data for the adsorption of nickel on TiO2 nanoparticles were tested with two adsorption isotherm models, the Langmuir and Freundlich equations. The Langmuir model fits the adsorption isotherm data of nickel better than the Freundlich model. The maximum adsorption capacity obtained using the Langmuir isotherm model is 1.115 mg g−1 at pH 4. The dimensionless equilibrium parameter, R L, signifies a favorable adsorption of nickel on TiO2 nanoparticles adsorbent, and was found to be between 0.354 (0 < R L < 1).












Similar content being viewed by others
References
C. Li, F. Xie, Y. Ma, T. Cai, H. Li, Z. Huang, G. Yuan, J. Hazard. Mater. 78, 823 (2010)
A. Nair, A.A. Juwarkar, S. Devotta, J. Hazard. Mater. 152, 545 (2008)
E. Malkoc, Y. Nuhoglu, J. Hazard. Mater. 127, 120 (2005)
P. Gomathi Priya, C.A. Bashab, V. Ramamurthia, S. Nathira Begum, J. Hazard. Mater. 163, 899 (2009)
K. Animes, S. Golder Varappurath, N. Dhaneesh Amar, R. Samanta Subhabrata, Chem. Eng. Technol. 31(1), 143 (2008)
P. Papachristou, K.J. Haralambous, M. Loizidou, N. Spyrellis, J. Environ. Sci. Heal. A. 28(1), 135 (1993)
A.M. El-Kamash, J. Hazard. Mater. 151, 432 (2008)
I. Villaescusa, N. Fiol, M. Martinez, N. Miralles, J. Poch, J. Serarols, Water Res. 38, 992 (2004)
L. Zhi-rong, Z. Shao-qi, Process Saf. Environ. 88, 62 (2010)
W. Qiu, Y. Zheng, Chem. Eng. J. 145, 483 (2009)
M. Fereidouni, A. Daneshi, H. Younesi, J. Hazard. Mater. 168, 1437 (2009)
A.L. Castro, M.R. Nunes, A.P. Carvalho, F.M. Costa, M.H. Floreˆncio, Solid State Sci. 10, 602 (2008)
B.E. Yoldas, J. Mater. Sci. 21, 1087 (1986)
Z. Ghasemi, A. Seif, T.S. Ahmadi, B. Zargar, F. Rashidi, G.M. Rouzbahani, Adv. Powder Technol. 23, 148–156 (2012)
S. Bang, M. Patel, L. Lippincott, X. Meng, Chemosphere 60(3), 389 (2005)
S. Kasap, H. Tel, S. Piskin, J. Radioanal. Nucl. Chem. 289, 489 (2011)
V. Belessi, G. Romanos, N. Boukos, D. Lambropoulou, C. Trapalis, J. Hazard. Mater. 170(2–3), 836 (2009)
M. Pirilä, M. Martikainen, K. Ainassaari, T. Kuokkanen, R.L. Keiski, J Colloid Interface Sci. 353, 257 (2011)
M. Sari Yilmaz, S. Kasap, O. Dere Ozdemir, S. Piskin, The removal of nickel from leachate of galvanic sludge with titanium dioxide. TMS 2011 Annual Meeting & Exhibition, Sandiego, USA, February 27–March 3 2011
X. Xie, L. Gao, Curr. Appl. Phys. 9(3), 185 (2009)
E.S. Abechi, C.E. Gimba, A. Uzairu, J.A. Kagbu, J. Appl. Sci. Res. 3(1), 154 (2011)
M.K. Aroua, S.P.P. Leong, L.Y. Teo, C.Y. Yin, W.M.A.W. Daud, Bioresour. Technol. 99(13), 5786 (2008)
M.A. Malana, S. Ijaz, M.N. Ashiq, Desalination 263, 249 (2010)
J. Mathew, S.H. Tandon, Can. J. Chem. 55(22), 3857 (1977)
V. Srihari, A. Das, Desalination 225, 220 (2008)
M. Rao, A.V. Parwate, A.G. Bhole, Waste Manage. 22, 821 (2002)
E. Metwally, T. El-Zakla, R.R. Ayoub, J. Nucl. Radiochem. Sci. 9(1), 1 (2008)
S.H. Chien, W.R. Clayton, Soil Sci. Soc. Am. J. 44(2), 265 (1980)
D. Mohan, K.P. Singh, Water Res. 36, 2304 (2002)
C.W. Cheung, C.F. Porter, G. McKay, J. Chem. Technol. Biotechnol. 75, 963 (2000)
P. Podkos′cielny, K. Nieszporek, J. Colloid Interface Sci. 354, 282 (2011)
N. Kannan, M. Meenakshi Sundaram, Dyes Pigment. 51, 25 (2001)
W.J. Weber, J.C. Morrıs, J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31 (1963)
S. Mellouk, A. Belhakem, K. Marouf-Khelifa, J. Schott, A. Khelifa, J. Colloid Interface Sci. 360, 716 (2011)
O. Abdelwahab, Egypt. J. Aquat. Res. 33(1), 125 (2007)
G. McKay, Y.S. Ho, Process Biochem. 34, 451 (1999)
I. Langmuir, J. Am. Chem. Soc. 38(11), 2221 (1916)
H.M.F. Freundlich, J. Phys. Chem. 57, 385 (1906)
N. Öztürk, T.E. Bektaş, J. Hazard. Mater. B112, 155 (2004)
X.S. Wang, Open Environ. Pollut. Toxicol. J. 1, 107 (2009)
S.R. Shukla, R.S. Pai, Bioresour. Technol. 96(13), 1430 (2005)
M. Torab-Mostaedi, H. Ghassabzadeh, M. Ghannadi-Maragheh, S.J. Ahmadi, H. Taheri, Braz. J. Chem. Eng. 27(2), 299 (2010)
B. Bayat, J. Hazard. Mater. 95, 251 (2002)
Y.S. Ho, J.F. Porter, G. Mckay, Water Air Soil Pollut. 141, 1 (2002)
K. Moodley, R. Singh, E.T. Musapatika, M.S. Onyango, A. Ochieng, Water SA. 37, 41 (2011)
J.A. Otun, I.A. Oke, N.O. Olarinoye, D.B. Adie, C.A. Okuofu, J. Appl. Sci. 6, 2368 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yilmaz, M.S., Ozdemir, O.D., Kasap, S. et al. The kinetics and thermodynamics of nickel adsorption from galvanic sludge leachate on nanometer titania powders. Res Chem Intermed 41, 1499–1515 (2015). https://doi.org/10.1007/s11164-013-1288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1288-8