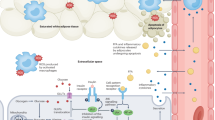
Abstract
Infertility is a global health problem affecting 10–15% of couples in reproductive age. Recent studies have provided growing evidence supporting that lifestyle factors can affect male fertility through alterations in endocrine profiles, spermatogenesis and/or sperm function. One of these critical factors could be the change in the food intake behavior in modern societies that produces metabolic alterations. Regarding this, metabolic syndrome (MetS) prevalence has increased in epidemic in the last 40–50 years. Although MetS is associated with advanced age, changes in lifestyles have accelerated the appearance of symptoms in the reproductive age. We review herein the current understanding of the relationship between MetS and the male reproductive status. For this purpose, in this narrative review a comprehensive literature search was made in both animal models and men, allowing us to evaluate such relationship. This analysis showed a high variability in the reproductive phenotypes observed in patients and mice suffering MetS, including sperm parameters, fertility and offspring health. In view of this, we proposed that the reproductive effects, which are diverse and not robust, observed among MetS-affected males, might depend on additional factors not associated with the metabolic condition and contributed not only by the affected male but also by his partner. With this perspective, this review provides a more accurate insight of this syndrome critical for the identification of specific diagnostic indicators and treatment of MetS-induced fertility disorders.


Similar content being viewed by others
Abbreviations
- HFD:
-
High-fat diet
- HPG:
-
Hypothalamic-pituitary–gonadal axis
- ICSI:
-
Intracytoplasmic sperm injection
- IL:
-
Interleukin
- MetS:
-
Metabolic syndrome
- MMP:
-
Mitochondrial membrane potential
- ROS:
-
Reactive oxygen species
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- ZP:
-
Zona pellucida
References
Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26.
Oehninger S, Ombelet W. Limits of current male fertility testing. Fertil Steril. Elsevier Inc.; 2019;111:835–41.
Hart K, Tadros NN. The role of environmental factors and lifestyle on male reproductive health, the epigenome, and resulting offspring. Panminerva Med. 2019;61:187–95.
Leisegang K, Dutta S. Do lifestyle practices impede male fertility? Andrologia: Blackwell Publishing Ltd; 2020.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation. International Circulation. 2009;120:1640–5.
de la Iglesia R, Loria-Kohen V, Zulet MA, Martinez JA, Reglero G, de Molina AR. Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int J Mol Sci. 2016;17.
Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–9.
Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JMA, Volle DH. Cholesterol and male fertility: What about orphans and adopted? Mol Cell Endocrinol. 2013;368:30–46.
Morrison CD, Brannigan RE. Metabolic syndrome and infertility in men. Best Pract Res Clin Obstet Gynaecol. Elsevier Ltd; 2015;29:507–15.
Crean AJ, Senior AM. High-fat diets reduce male reproductive success in animal models: A systematic review and meta-analysis. Obes. Rev. Blackwell Publishing Ltd; 2019. p. 921–33.
Martins AD, Majzoub A, Agawal A. Metabolic syndrome and male fertility. World J Mens Health. 2019;37:113–27.
Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum Reprod Update Oxford University Press. 2018;24:86–105.
Marchiani S, Vignozzi L, Filippi S, Gurrieri B, Comeglio P, Morelli A, et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: Relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol Elsevier Ireland Ltd. 2015;401:12–24.
Cui X, Long C, Tian J, Zhu J. Protective Effects of Fluvastatin on Reproductive Function in Obese Male Rats Induced by High-Fat Diet through Enhanced Signaling of mTOR. Cell Physiol Biochem S Karger AG. 2017;41:598–608.
Ferramosca A, Conte A, Moscatelli N, Zara V. A high-fat diet negatively affects rat sperm mitochondrial respiration. Andrology Blackwell Publishing Ltd. 2016;4:520–5.
Ferramosca A, Moscatelli N, Di Giacomo M, Zara V. Dietary fatty acids influence sperm quality and function. Andrology Blackwell Publishing Ltd. 2017;5:423–30.
Alhashem F, Alkhateeb M, Alshahrani M, Elrefaey H, Alsunaidi M, Alessa R, et al. Exercise protects against obesity induced semen abnormalities via downregulating stem cell factor, upregulating Ghrelin and normalizing oxidative stress. EXCLI J. 2014;13:551–72.
Li Y, Liu L, Wang B, Xiong J, Li Q, Wang J, et al. Impairment of reproductive function in a male rat model of non-alcoholic fatty liver disease and beneficial effect of N-3 fatty acid supplementation. Toxicol Lett. 2013;222:224–32.
Kaku K, Fiedorek FT, Province M, Permutt A. Genetic Analysis of Glucose Tolerance in Inbred Mouse Strains Evidence for Polygenic Control. Diabetes. 1988;37:707–13.
Kooptiwut S, Zraika S, Thorburn AW, Dunlop ME, Darwiche R, Kay TW, et al. Comparison of insulin secretory function in two mouse models with different susceptibility to β-cell failure. Endocrinology. 2002;143:2085–92.
Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential Effects of Fat and Sucrose on the Development of Obesity and Diabetes in C57BL/6J and A/J Mice. Metabolism. 1995;44:645–51.
Black BL, Croom J, Eisen EJ, Petro AE, Edwards CL, Surwit RS. Differential Effects of Fat and Sucrose on Body Composition in A/J and C57BL/6 Mice. Metabolism. 1998;47:1354–9.
Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, Carbohydrate, and Calories in the Development of Diabetes and Obesity in the C57BL/6J Mouse. Metabolism. W.B. Saunders; 2004;53:454–7.
Surwit RS, Kuhn CM, Cochrane C, Mccubbin JA, Feinglos MN. Diet-Induced Type II Diabetes in C57BL/6J Mice. Diabetes. 1988;37:1163–7.
Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of Expression of Insulin Resistance and Hyperglycemia by Different Genetic Factors in Diabetic C57BL/6J Mice. Diabetes. 1991;40:82–7.
Burcelin RM, Crivelli V, Dacosta A, Roy-tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Metab. 2002;282:834–42.
Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Granner DK, Wasserman DH. Hexokinase II Overexpression Improves Exercise-Stimulated But Not Insulin-Stimulated Muscle Glucose Uptake in High-Fat-Fed C57BL/6J Mice. Diabetes. 2004;53:306–14.
Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–10.
Palmer NO, Fullston T, Mitchell M, Setchell BP, Lane M. SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reprod Fertil Dev. 2011;23:929–39.
Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol - Endocrinol Metab. 2012;302:768–80.
Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. Public Library of Science; 2015;10.
Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, Lane M. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep. American Physiological Society; 2015;3.
Gómez-Elías MD, Rainero Cáceres TS, Giaccagli MM, Guazzone VA, Dalton GN, De Siervi A, et al. Association between high-fat diet feeding and male fertility in high reproductive performance mice. Sci Rep. Nature Research; 2019;9.
McPherson N, Lane M. Metformin treatment of high-fat diet-fed obese male mice restores sperm function and fetal growth, without requiring weight loss. Asian J Androl. 2020;22:560–8.
McPherson NO, Fullston T, Bakos HW, Setchell BP, Lane M. Obese father’s metabolic state, adiposity, and reproductive capacity indicate son’s reproductive health. Fertil Steril. Elsevier Inc.; 2014;101:865–73.
Dupont C, Faure C, Daoud F, Gautier B, Czernichow S, Lévy R, et al. Metabolic syndrome and smoking are independent risk factors of male idiopathic infertility. Basical Clin Androl. Basic and Clinical Andrology; 2019;29.
Ventimiglia E, Capogrosso P, Colicchia M, Boeri L, Serino A, Castagna G, et al. Metabolic syndrome in white European men presenting for primary couple’s infertility: investigation of the clinical and reproductive burden. Andrology. 2016;4:944–51.
Elenkov A, Al-Jebari Y, Giwercman A. More Prevalent Prescription of Medicine for Hypertension and Metabolic Syndrome in Males from Couples Undergoing Intracytoplasmic Sperm Injection. Sci Rep. Springer: US; 2018. p. 8.
Ehala-Aleksejev K, Punab M. The effect of metabolic syndrome on male reproductive health: A cross-sectional study in a group of fertile men and male partners of infertile couples. PLoS One. 2018;13.
Lotti F, Corona G, Vignozzi L, Rossi M, Maseroli E, Cipriani S, et al. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl. 2014;16:295–304.
Pilatz A, Hudemann C, Wolf J, Halefeld I, Paradowska-Dogan A, Schuppe HC, et al. Metabolic syndrome and the seminal cytokine network in morbidly obese males. Andrology. 2017;5:23–30.
Le MT, Nguyen DN, Le DD, Tran NQT. Impact of body mass index and metabolic syndrome on sperm DNA fragmentation in males from infertile couples: A cross-sectional study from Vietnam. Metab Open. Elsevier BV; 2020;7.
Elfassy Y, Bongrani A, Levy P, Foissac F, Fellahi S, Faure C, et al. Relationships between Metabolic Status, Seminal Adipokines, and Reproductive Functions in Men from Infertile Couples. Eur J Endocrinol. 2020;182:67–77.
Leisegang K, Udodong A, Bouic PJD, Henkel RR. Effect of the metabolic syndrome on male reproductive function: A case-controlled pilot study. Andrologia. 2014;46:167–76.
Leisegang K, Bouic PJD, Henkel RR. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am J Reprod Immunol. 2016;76:155–63.
Rosety I, Elosegui S, Pery MT, Fornieles G, Rosety JM, DÍaz AJ, et al. Association between abdominal obesity and seminal oxidative damage in adults with metabolic syndrome. Rev Med Chil. 2014;142:732–7.
McPherson NO, Tremellen K. Increased BMI ‘alone’ does not negatively influence sperm function - a retrospective analysis of men attending fertility treatment with corresponding liver function results. Obes Res Clin Pract. Asia Oceania Assoc. for the Study of Obesity; 2020;14:164–7.
Saikia UK, Saikia K, Sarma D, Appaiah S. Sertoli Cell Function in Young Males with Metabolic Syndrome. Indian J Endocrinol Metab. 2019;23:251–6.
Chen YY, Kao TW, Peng TC, Yang HF, Chen-Jung WU, Chen WL. Metabolic syndrome and semen quality in adult population. J Diabetes. 2019;12:294–304.
Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013;1:229–39.
Elsamanoudy AZ, Abdalla HA, Hassanien M, Gaballah MA. Spermatozoal cell death-inducing DNA fragmentation factor-α-like effector A (CIDEA) gene expression and DNA fragmentation in infertile men with metabolic syndrome and normal seminogram. Diabetol Metab Syndr. BioMed Central; 2016;8.
Ventimiglia E, Capogrosso P, Serino A, Boeri L, Colicchia M, La Croce G, et al. Metabolic syndrome in White-European men presenting for secondary couple’s infertility: An investigation of the clinical and reproductive burden. Asian J Androl. 2017;18:368–73.
Zhao L, Pang A. Effects of Metabolic Syndrome on Semen Quality and Circulating Sex Hormones: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2020;11.
Leisegang K, Henkel R, Agarwal A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am J Reprod Immunol. 2019;82.
Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. Elsevier Inc.; 2013;62:457–78.
Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and Nutritional Factors in Male (In)fertility—Underestimated Factors. J Clin Med. MDPI AG; 2020;9.
Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–67.
Schoeller EL, Schon S, Moley KH. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012;349:839–47.
Manfredi-Lozano M, Roa J, Tena-Sempere M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol. Academic Press Inc.; 2018;48:37–49.
Tarleton GW, Gondos B, Formby B. Testicular Alterations in the Nonobese Diabetic Mouse. Endocr Pathol. 1990;1:85–93.
Alves MG, Martins AD, Cavaco JE, Socorro S, Oliveira PF. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers. Informa UK Limited; 2013;1.
Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: An evidence based review. Int J Reprod BioMed. 2016;14:729–36.
Mallidis C, Czerwiec A, Filippi S, O’Neill J, Maggi M, McClure N. Spermatogenic and sperm quality differences in an experimental model of metabolic syndrome and hypogonadal hypogonadism. Reproduction. 2011;142:63–71.
Funes A, Saez Lancellotti TE, Santillan LD, Della Vedova MC, Monclus MA, Cabrillana ME, et al. A chronic high-fat diet causes sperm head alterations in C57BL/6J mice. Heliyon. Elsevier BV; 2019;5.
Martínez P, Proverbio F, Camejo MI. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J Androl. 2007;9:102–7.
Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–35.
Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, et al. Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol. 2018;9.
Morelli A, Filippi S, Comeglio P, Sarchielli E, Cellai I, Pallecchi M, et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am J Physiol - Endocrinol Metab. 2019;316:519–35.
Vignozzi L, Filippi S, Comeglio P, Cellai I, Sarchielli E, Morelli A, et al. Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: An experimental study in the rabbit. Mol Cell Endocrinol Elsevier Ireland Ltd. 2014;384:143–54.
Crisóstomo L, Rato L, Jarak I, Silva BM, Raposo JF, Batterham RL, et al. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction. 2019;158:377–87.
Oliveira PF, Sousa M, Silva BM, Monteiro MP, Alves MG. Obesity, energy balance and spermatogenesis. Reproduction. BioScientifica Ltd.; 2017. p. 173–85.
Lee Y, Dang JT, Switzer N, Yu J, Tian C, Birch DW, et al. Impact of Bariatric Surgery on Male Sex Hormones and Sperm Quality: a Systematic Review and Meta-Analysis. Obes. Surg. Springer: New York LLC; 2019. p. 334–46.
Bertoldo MJ, Guibert E, Tartarin P, Guillory V, Froment P. Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology. Elsevier Inc.; 2014;68:262–8.
Zhou J, Massey S, Story D. Li L. Metformin: An old drug with new applications. Int J Mol Sci; 2018. p. 19.
Arabey EL, AA, Abdalla M, Eltayb WA. Metformin: Ongoing journey with superdrug revolution. Adv Pharm Bull. 2019;9:1–4.
Bosman E, Esterhuizen AD, Rodrigues FA, Becker PJ, Hoffmann WA. Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males. Andrologia. 2015;47:974–9.
Rago V, De Rose D, Santoro M, Panza S, Malivindi R, Andò S, et al. Human sperm express the receptor for glucagon-like peptide-1 (GLP-1) which affects sperm function and metabolism. Endocrinology. 2020;161.
Zhang E, Xu F, Liang H, Yan J, Xu H, Li Z, et al. GLP-1 Receptor Agonist Exenatide Attenuates the Detrimental Effects of Obesity on Inflammatory Profile in Testis and Sperm Quality in Mice. Am J Reprod Immunol. Blackwell Publishing Ltd; 2015;74:457–66.
Montanino Oliva M, Minutolo E, Lippa A, Iaconianni P, Vaiarelli A. Effect of Myoinositol and Antioxidants on Sperm Quality in Men with Metabolic Syndrome. Int J Endocrinol. Hindawi Publishing Corporation; 2016;2016.
Zhou X, Zhai S. Effect of L-carnitine and/or L-acetyl-carnitine in nutrition treatment for male infertility: a systematic review. Asia Pac J Clin Nutr. 2007.
Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science (80- ). 2015;350.
Lambrot R, Xu C, Chountalos G, Cohen T, Paquet M, Suderman M, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Comun. 2013;4.
Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science (80- ). 2014;345.
Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, et al. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013;11.
Schagdarsurengin U, Steger K. Epigenetics in male reproduction: Effect of paternal diet on sperm quality and offspring health. Nat Rev Urol Nature Publishing Group. 2016;13:584–95.
Martin-DeLeon PA. Epididymosomes: Transfer of fertility-modulating proteins to the sperm surface. Asian J Androl. 2015;17:720–5.
Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, et al. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev Cell. Elsevier Inc.; 2018;46:481–94.
Ng SF, Lin RCY, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β 2-cell dysfunction in female rat offspring. Nature Nature Publishing Group. 2010;467:963–6.
Lecomte V, Maloney CA, Wang KW, Morris MJ. Effects of paternal obesity on growth and adiposity of male rat offspring. Am J Physiol - Endocrinol Metab. 2017;312:117–25.
Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27:1391–400.
Fullston T, Teague EMCO, Palmer NO, Deblasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–43.
McPherson NO, Owens JA, Fullston T, Lane M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol - Endocrinol Metab. 2015;308:805–21.
McPherson NO, Lane M, Sandeman L, Owens JA, Fullston T. An exercise‐only intervention in obese fathers restores glucose and insulin regulation in conjunction with the rescue of pancreatic islet cell morphology and microRNA expression in male offspring. Nutrients. 2017;9.
Ornellas F, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Programming of obesity and comorbidities in the progeny: Lessons from a model of diet-induced obese parents. PLoS One. 2015;10.
Sanchez-Garrido MA, Ruiz-Pino F, Velasco I, Barroso A, Fernandois D, Heras V, et al. Intergenerational Influence of paternal obesity on metabolic and reproductive health parameters of the offspring: Male-preferential impact and involvement of kiss1-mediated pathways. Endocrinology. 2018;159:1005–18.
de Jesus DF, Orime K, Kaminska D, Kimura T, Basile G, Wang CH, et al. Parental metabolic syndrome epigenetically reprograms offspring hepatic lipid metabolism in mice. J Clin Invest. 2020;130:2391–404.
Grandjean V, Fourré S, De Abreu DAF, Derieppe MA, Remy JJ, Rassoulzadegan M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep. Nature Publishing Group; 2015;14.
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science (80- ). 2016;351:397–400.
Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science (80- ). 2016;351:391–6.
Rassoulzadegan M, Cuzin F. Nutrition meets heredity: A case of RNA-mediated transmission of acquired characters. Environ Epigenetics Oxford University Press (OUP). 2018;4:1–4.
Sinclair KD, Watkins AJ. Parental diet, pregnancy outcomes and offspring health: Metabolic determinants in developing oocytes and embryos. Reprod Fertil Dev. 2013;26:99–114.
Huypens P, Sass S, Wu M, Dyckhoff D, Tschöp M, Theis F, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet. 2016;48:497–9.
Sabo RT, Lu Z, Deng X, Ren C, Daniels S, Arslanian S, et al. Parental and offspring associations of the metabolic syndrome in the Fels Longitudinal Study. Am J Clin Nutr. 2012;96:461–6.
Lee K. Metabolic syndrome in Korean adolescents and young adult offspring and their parents. Asia Pac J Clin Nutr. 2017;26:713–8.
Luo BF, Du L, Li JX, Pan BY, Xu JM, Chen J, et al. Heritability of metabolic syndrome traits among healthy younger adults: A population based study in China. J Med Genet. 2010;47:415–20.
Klijs B, Angelini V, Mierau JO, Smidt N. The role of life-course socioeconomic and lifestyle factors in the intergenerational transmission of the metabolic syndrome: Results from the LifeLines Cohort Study. Int J Epidemiol. 2016;45:1236–46.
Linares Segovia B, Gutiérrez Tinoco M, Izquierdo Arrizon A, Guízar Mendoza JM, Amador Licona N. Long-term consequences for offspring of paternal diabetes and metabolic syndrome. Exp Diabetes Res. 2012;2012.
Baxi R, Vasan SK, Hansdak S, Samuel P, Jeyaseelan V, Geethanjali FS, et al. Parental determinants of metabolic syndrome among adolescent Asian Indians: A cross-sectional analysis of parent–offspring trios. J Diabetes. 2016;8:494–501.
Khan RJ, Gebreab SY, Riestra P, Xu R, Davis SK. Parent-offspring association of metabolic syndrome in the Framingham Heart Study. Diabetol Metab Syndr. 2014;6.
Azizi F, Farahani ZK, Ghanbarian A, Sheikholeslami F, Mirmiran P, Momenan AA, et al. Familial aggregation of the metabolic syndrome: Tehran lipid and glucose study. Ann Nutr Metab. 2009;54:189–96.
Zauner G, Girardi G. Potential causes of male and female infertility in Qatar. J Reprod Immunol. Elsevier; 2020;141.
Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril. 2005;84:919–24.
Jensen TK, Swan S, Jørgensen N, Toppari J, Redmon B, Punab M, et al. Alcohol and male reproductive health: A cross-sectional study of 8344 healthy men from Europe and the USA. Hum Reprod Oxford University Press. 2014;29:1801–9.
Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nat. Metab. Nature Research; 2019. p. 34–46.
Tomaiuolo R, Veneruso I, Cariati F. D’argenio V. Microbiota and human reproduction: The case of male infertility. High-Throughput. MDPI AG; 2020. p. 9.
Koedooder R, Mackens S, Budding A, Fares D, Blockeel C, Laven J, et al. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum Reprod Update Oxford University Press. 2019;25:298–325.
Ding N, Zhang X, Di ZX, Jing J, Liu SS, Mu YP, et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut BMJ Publishing Group. 2020;69:1608–19.
Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–41.
Montagutelli X. Effect of the Genetic Background on the Phenotype of Mouse Mutations. J Am Soc Nephrol. 2000;11:101–5.
Riordan JD, Nadeau JH. From Peas to Disease: Modifier Genes, Network Resilience, and the Genetics of Health. Am J Hum Genet Cell Press. 2017;101:177–91.
Nayernia K, Adham IM, Burkhardt-Göttges E, Neesen J, Rieche M, Wolf S, et al. Asthenozoospermia in Mice with Targeted Deletion of the Sperm Mitochondrion-Associated Cysteine-Rich Protein (Smcp) Gene. Mol Cell Biol. American Society for Microbiology; 2002;22:3046–52.
Weigel Muñoz M, Battistone MA, Carvajal G, Maldera JA, Curci L, Torres P, et al. Influence of the genetic background on the reproductive phenotype of mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Biol Reprod Oxford University Press. 2018;99:363–83.
Tortoriello DV, McMinn J, Chua SC. Dietary-Induced Obesity and Hypothalamic Infertility in Female DBA/2J Mice. Endocrinology. 2004;145:1238–47.
Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56:1129–39.
McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141:210–7.
Patel DP, Jenkins TG, Aston KI, Guo J, Pastuszak AW, Hanson HA, et al. Harnessing the full potential of reproductive genetics and epigenetics for male infertility in the era of “big data.” Fertil Steril. 2020;113:478–88.
Jenkins TG, Aston KI, Hotaling JM, Shamsi MB, Simon L, Carrell DT. Teratozoospermia and asthenozoospermia are associated with specific epigenetic signatures. Andrology. 2016;4:843–9.
Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26:2558–69.
Jodar M, Sendler E, Moskovtsev SI, Librach CL, Goodrich R, Swanson S, et al. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci Transl Med. 2015;7.
Jokiniemi A, Kuusipalo L, Ritari J, Koskela S, Partanen J, Kekäläinen J. Gamete-level immunogenetic incompatibility in humans–towards deeper understanding of fertilization and infertility? Heredity (Edinb). Springer Nature. 2020;125:281–9.
Funding
This work was supported by the National Agency for Scientific and Technological Promotion (PICT 2017–668) and the National Research Council (PUE 2017) of Argentina.
Author information
Authors and Affiliations
Contributions
DJC and VGD: conceptualization, data curation, funding acquisition, investigation, writing—original draft, review & editing. MMG, JDH and LNG: data curation, investigation, visualization, writing—original draft. PSC: investigation, writing—original draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, D.J., Giaccagli, M.M., Herzfeld, J.D. et al. Metabolic syndrome and male fertility disorders: Is there a causal link?. Rev Endocr Metab Disord 22, 1057–1071 (2021). https://doi.org/10.1007/s11154-021-09659-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09659-9