Abstract
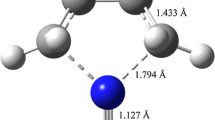
The β-elimination kinetics of 2,2-dihaloethyltrihalosilanes in the gas phase has been studied computationally using density functional theory (DFT) along with the M06-2x exchange–correlation functional and the aug-cc-pVTZ basis set. The calculated energy profiles have been supplemented with calculations of rate constants under atmospheric pressure and in the fall-off regime, by means of transition state theory (TST), variational transition state theory (VTST), and statistical Rice–Ramsperger–Kassel–Marcus (RRKM) theory. Activation energies and rate constants obtained using the M06-2x/aug-cc-pVTZ approaches are in good agreement with the available experimental data. Analysis of bond order, natural bond orbitals, and synchronicity parameters suggests that the β-elimination of the studied compounds can be described as concerted and slightly asynchronous. The transition states of these reactions correspond to four-membered cyclic structures. Based on the optimized ground state geometries, a natural bond orbital (NBO) analysis of donor–acceptor interactions also show that the resonance energies related to the electronic delocalization from \(\sigma_{{{\text{C}}_{ 1} {-}{\text{C}}_{ 2} }}\) bonding orbitals to \(\sigma^{*}_{{{\text{C}}_{ 2} - {\text{Si}}_{ 3} }}\) antibonding orbitals, increase from 2,2-difluoroethyltrifluorosilane to 2,2-dichloroethyltrichlorosilane and then to 2,2-dibromoethyltriboromosilane. The decrease of \(\sigma_{{{\text{C}}_{ 1} {-}{\text{C}}_{ 2} }}\) bonding orbitals occupancies and increase of the \(\sigma^{*}_{{{\text{C}}_{ 2} - {\text{Si}}_{ 3} }}\) antibonding orbitals occupancies through \(\sigma_{{{\text{C}}_{ 1} - {\text{C}}_{ 2} }} \to \sigma^{*}_{{{\text{C}}_{ 2} - {\text{Si}}_{ 3} }}\) delocalizations could facilitate the β-elimination of the 2,2-difluoroethyltrifluorosilane compound, compared to 2,2-dichloroethyltrichlorosilane and 2,2-dibromoethyltriboromosilane.



Similar content being viewed by others
References
Haszeldine RN, Robinson PJ, Simmons RF (1964) J Chem Soc 0:1890
Eaborn C (1960) Organosilicon compounds. Butterworths Scientific Publications, Ltd., London
Haszeldine RN, Young JC (1959) Proc Chem Soc 394
Bevan WI, Haszeldine RN, Young JC (1961) Chem & Ind (London) 789
Haszeldine RN, Newlands MJ, Plumb JB (1960) Proc Chem Soc 4:147
Bell TN, Haszeldine RN, Newlands MJ, Plumb JB (1965) J Chem Soc 0:2107
Haszeldine RN, Robinson PJ, Simmons RF (1967) J Chem Soc B 1357
Davidson IMT, Eaborn C, Lilly MN (1964) J Chem Soc 2624
Davidson IMT, Metcalfe CJL (1964) J Chem Soc 2630
Davidson IMT, Jones MR (1965) J Chem Soc 5481
Davidson IMT, Jones MR, Pett C (1967) J Chem Soc B 937
Fishwick G, Haszeldine RN, Parkinson C, Robinson PJ, Simmons RF (1965) J Chem Soc Chem Commun 382
Eyring H (1935) J Chem Phys 3:107
Johnston HS (1966) Gas phase reaction rate theory. Roland Press, New York
Laidler KJ (1969) Theories of chemical reaction rates. McGraw-Hill, New York
Weston RE, Schwartz HA (1972) Chemical kinetics. Prentice-Hall, New York
Rapp D (1972) Statistical mechanics. Holt, Rinehart, and Winston, New York
Nikitin EE (1974) Theory of elementary atomic and molecular processes in gases. Clarendon Press, Oxford
Smith IWM (1980) Kinetics and dynamics of elementary gas reactions. Butterworths, London
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Vanden-Eijnden E, Tal FA (2005) J Chem Phys 123:184103
Garret BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730
Garret BC, Truhlar DG (1979) J Am Chem Soc 101:4534
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Dunning TH Jr (1989) J Chem Phys 90:1007
Robinson PJ, Holbrook KA (1972) Unimolecular reactions. Wiley, New York
Steinfeld JI, Francisco JS, Hase WL (1999) Chemical kinetics and dynamics. Prentice-Hall, Englewood Cliffs
Eyring H, Lin SH, Lin SM (1980) Basic chemical kinetics. Wiley, New York
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735
Badenhoop JK, Weinhold F (1999) Int J Quantum Chem 72:269
Knippenberg S, Bohnwagner MV, Harbach PH, Dreuw A (2015) J Phys Chem A 119:1323
Frenette M, Hatamimoslehabadi M, Bellinger-Buckley S, Laoui S, La J, Bag S, Mallidi S, Hasan T, Bouma B, Yelleswarapu C, Rochford J (2014) J Am Chem Soc 136:15853
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian Inc., Wallingford
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Chang R (2005) Physical chemistry for the biosciences. University Science Books, Sausalito
Moore JW, Pearson RG (1981) Kinetics and mechanism—The study of homogeneous chemical reactions. Wiley, New York
Carstensen HH, Dean AM, Deutschmann O (2007) Proc Combust Inst 31:149
Eckart C (1930) Phys Rev 35:1303
Johnson HS, Heicklen J (1962) J Phys Chem 66:532
Canneaux S, Bohr F, Henon E (2014) J Comput Chem 35:82
Shiroudi A, Zahedi E (2016) RSC Adv 6:91882
Marquez E, Mora JR, Cordova T, Chuchani G (2009) J Phys Chem A 113:2600
Troe J (1977) J Chem Phys 66:4758
Mourits FM, Rummens HA (1977) Can J Chem 55:3007
Kee RJ, Rupley FM, Miller JA, Coltrin ME, Grcar JF, Meeks E, Moffat HK, Lutz AE, Dixon-Lewis G, Smooke MD, Warnatz J, Evans GH, Larson RS, Mitchell RE, Petzold LR, Reynolds WC, Caracotsios M, Stewart WE, Glarborg P, Wang C, McLellan CL, Adigun O, Houf WG, Chou CP, Miller SF, Ho P, Young PD, Young DJ, Hodgson DW, Petrova MV, Puduppakkam KV (2010) CHEMKIN. Reaction Design Inc., San Diego
Wigner E (1937) J Chem Phys 5:720
Horiuti J (1938) Bull Chem Soc Jpn 13:210
Keck JC (1960) J Chem Phys 32:1035
Keck JC (1967) Adv Chem Phys 13:85
Truhlar DG, Garrett BC (1984) Annu Rev Phys Chem 35:159
Garrett BC, Truhlar DG (1979) J Phys Chem 83:1052
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440
Evans MG, Polanyi M (1938) Trans Faraday Soc 34:11
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transition state theory. In: Baer M (ed) Theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, pp 65–137
Baer T, Hase WL (1996) Unimolecular reaction dynamics. Oxford University Press, Oxford
Garrett BC, Truhlar DG (1979) J Phys Chem 83:1079
Gilbert RG, Smith SC (1990) Theory of unimolecular and recombination reactions. Blackwell Scientific, Oxford
Klippenstein SJ, Yang Y-C, Ryzhov V, Dunbar RC (1996) J Chem Phys 104:4502
Wardlaw DM, Marcus RA (1988) Adv Chem Phys 70:231
Hase WL, Wardlaw DM (1989) In Ashfold MNR, Baggott JE (eds) Advances in gas-phase photochemistry and kinetics: bimolecular collisions. Royal Society, London, p 171
Klippenstein SJ (1995) In: Liu K, Wagner AF (eds) Advances in physical chemistry: the chemical dynamics and kinetics of small radicals, vol 1. World scientific, Singapore
Taatjes CA, Klippenstein SJ (2001) J Phys Chem A 105:8567
Isaacson AD, Truhlar DG, Rai SN, Steckler R, Hancock GC, Garrett BC, Redmon M (1987) J Comput Phys Commun 47:91
Borisov YA, Arcia EE, Mielke SL, Garrett BC, Dunning TH Jr (2001) J Phys Chem A 105:7724
Hammond GS (1953) J Am Chem Soc 77:334
Agmon N, Levine RD (1977) Chem Phys Lett 52:197
Maccoll A (1958) J Chem Soc 3398
Sun W, Yang L, Yu L, Saeys M (2009) J Phys Chem A 113:7852
Lendvay G (1989) J Phys Chem 93:4422
Wiberg KB (1968) Tetrahedron 24:1083
Reed E, Carpenter JE, Weinhold F, NBO version 3.1
Moyano A, Periclas MA, Valenti E (1989) J Org Chem 54:573
Rosas F, Dominguez RM, Tosta M, Mora JR, Marquez E, Cordova T, Chuchani G (2010) J Phys Org Chem 23:743
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Carpenter JE, Weinhold F (1988) J Mol Struct (Theochem) 169:41
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11144_2017_1332_MOESM1_ESM.pdf
Supplementary material 1 (PDF 105 kb). Table S1 Unimolecular rate constants for all reaction steps involved in the reported chemical pathways (results obtained by means of RRKM theory at different pressures and temperatures, according to the computed M06-2x/aug-cc-pVTZ energy profiles)
Rights and permissions
About this article
Cite this article
Oliaey, A.R., Shiroudi, A., Zahedi, E. et al. Theoretical study on the mechanisms and kinetics of the β-elimination of 2,2-dihaloethyltrihalosilanes (X = F, Cl, Br) compounds: a DFT study along with a natural bond orbital analysis. Reac Kinet Mech Cat 124, 27–44 (2018). https://doi.org/10.1007/s11144-017-1332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1332-6