Abstract
Purpose
While some work has been done on Health-Related Quality of Life (HRQoL) in statin users, none has focused specifically on statin-associated muscle symptoms (SAMS) sufferers. The objective was to assess self-reported HRQoL, before and after statin withdrawal, in patients reporting SAMS. We hypothesized that the presence of SAMS associated with decreased self-reported physical and mental well-being.
Methods
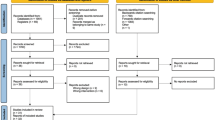
Patients (50 men/28 women [M/W], aged 49 ± 9 years [Mean ± SD]) in primary cardiovascular prevention were recruited into three cohorts: statin users with (SAMS, 29 M/18W) or without symptoms (No SAMS, 10 M/5W) and controls (11 M/5W). The Short Form 36 Health Survey (SF-36) was used to assess HRQoL. All variables were measured before and after 2 months of statin withdrawal, and repeated measures analyses were used to verify withdrawal and group effects as well as their interaction.
Results
SF-36 physical and mental component scores (respectively, PCS and MCS) were lower in the SAMS group compared with other groups (both p < 0.01). Statin withdrawal led to an increase in LDL cholesterol for statin users (+69.0%, p < 0.01) and an improvement in well-being in the SAMS group, other groups showing no change. A time x category interaction (p = 0.02) was seen for PCS and post hoc analyses showed that statin withdrawal improved PCS and MCS (respectively, +12.5% [ES 0.77] and +5.1% [ES 0.27], both p < 0.05) in the SAMS group.
Conclusion
Patients self-reporting SAMS showed improved HRQoL following drug withdrawal, but this was mirrored by a rise in LDL cholesterol. These findings should be considered by clinicians in the evaluation and follow-up of treatment with statins.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author [DRJ].
References
Cohen, J. D., Brinton, E. A., Ito, M. K., & Jacobson, T. A. (2012). Understanding Statin use in America and gaps in patient Education (USAGE): an internet-based survey of 10138 current and former statin users. Journal of Clinical Lipidology, 6(3), 208–215. https://doi.org/10.1016/j.jacl.2012.03.003
Sivashanmugarajah, A., Fulcher, J., Sullivan, D., Elam, M., Jenkins, A., & Keech, A. (2019). Suggested clinical approach for the diagnosis and management of ‘statin intolerance’ with an emphasis on muscle-related side-effects. Internal Medicine Journal, 49(9), 1081–1091. https://doi.org/10.1111/imj.14429
Ridker, P. M., Danielson, E., Fonseca, F. A., Genest, J., Gotto, A. M., Jr., Kastelein, J. J., Koenig, W., Libby, P., Lorenzatti, A. J., MacFadyen, J. G., Nordestgaard, B. G., Shepherd, J., Willerson, J. T., & Glynn, R. J. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine, 359(21), 2195–2207. https://doi.org/10.1056/NEJMoa0807646
Thompson, P. D., Panza, G., Zaleski, A., & Taylor, B. (2016). Statin-associated side effects. Journal of the American College of Cardiology, 67(20), 2395–2410. https://doi.org/10.1016/j.jacc.2016.02.071
Wagstaff, L. R., Mitton, M. W., Arvik, B. M., & Doraiswamy, P. M. (2003). Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 23(7), 871–880. https://doi.org/10.1592/phco.23.7.871.32720
Bruckert, E., Hayem, G., Dejager, S., Yau, C., & Begaud, B. (2005). Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovascular Drugs and Therapy, 19(6), 403–414. https://doi.org/10.1007/s10557-005-5686-z
Selva-O’Callaghan, A., Alvarado-Cardenas, M., Pinal-Fernandez, I., Trallero-Araguas, E., Milisenda, J. C., Martinez, M. A., Marin, A., Labrador-Horrillo, M., Juarez, C., & Grau-Junyent, J. M. (2018). Statin-induced myalgia and myositis: An update on pathogenesis and clinical recommendations. Expert Review of Clinical Immunology, 14(3), 215–224. https://doi.org/10.1080/1744666X.2018.1440206
Stroes, E. S., Thompson, P. D., Corsini, A., Vladutiu, G. D., Raal, F. J., Ray, K. K., Roden, M., Stein, E., Tokgozoglu, L., Nordestgaard, B. G., Bruckert, E., De Backer, G., Krauss, R. M., Laufs, U., Santos, R. D., Hegele, R. A., Hovingh, G. K., Leiter, L. A., Mach, F., … P European Atherosclerosis Society Consensus. (2015). Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis Society consensus panel statement on assessment aetiology and management. European Heart Journal, 36(17), 1012–1022. https://doi.org/10.1093/eurheartj/ehv043
Bouitbir, J., Sanvee, G. M., Panajatovic, M. V., Singh, F., & Krahenbuhl, S. (2020). Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacological Research, 154, 104201. https://doi.org/10.1016/j.phrs.2019.03.010
Taylor, B. A., & Thompson, P. D. (2018). Statin-associated muscle disease: Advances in diagnosis and management. Neurotherapeutics, 15(4), 1006–1017. https://doi.org/10.1007/s13311-018-0670-z
Turner, R. M., & Pirmohamed, M. (2019). Statin-related myotoxicity: A comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. Journal of Clinical Medicine, 9, 1. https://doi.org/10.3390/jcm9010022
Blacher, J., Bruckert, E., Farnier, M., Ferrieres, J., Henry, P., Krempf, M., & Mourad, J. J. (2019). Myalgia and statins: Separating the true from the false. La Presse Médicale, 48(10), 1059–1064. https://doi.org/10.1016/j.lpm.2019.07.034. (Myalgies et statines : demeler le vrai du faux.).
Hauser, W., Hansen, E., & Enck, P. (2012). Nocebo phenomena in medicine: their relevance in everyday clinical practice. Deutsches Ärzteblatt international, 109(26), 459–465. https://doi.org/10.3238/arztebl.2012.0459
Pedro-Botet, J., Climent, E., & Benaiges, D. (2019). Muscle and statins from toxicity to the nocebo effect. Expert Opinion on Drug Safety, 18(7), 573–579. https://doi.org/10.1080/14740338.2019.1615053
Robinson, J. G. (2019). New insights into managing symptoms during statin therapy. Progress in Cardiovascular Diseases, 62(5), 390–394. https://doi.org/10.1016/j.pcad.2019.10.005
Alonso, R., Cuevas, A., & Cafferata, A. (2019). Diagnosis and management of statin intolerance. Journal of Atherosclerosis and Thrombosis, 26(3), 207–215. https://doi.org/10.5551/jat.RV17030
Rosenson, R. S., Miller, K., Bayliss, M., Sanchez, R. J., Baccara-Dinet, M. T., Chibedi-De-Roche, D., Taylor, B., Khan, I., Manvelian, G., White, M., & Jacobson, T. A. (2017). The statin-associated muscle symptom clinical index (SAMS-CI): Revision for clinical use, content validation, and inter-rater reliability. Cardiovascular Drugs and Therapy, 31(2), 179–186. https://doi.org/10.1007/s10557-017-6723-4
Carlsson, C. M., Papcke-Benson, K., Carnes, M., McBride, P. E., & Stein, J. H. (2002). Health-related quality of life and long-term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs & aging, 19(10), 793–805. https://doi.org/10.2165/00002512-200219100-00008
Hyttinen, L., Kekalainen, P., Vuorio, A. F., Sintonen, H., & Strandberg, T. E. (2008). Health-related quality of life in elderly patients with familial hypercholesterolemia. International Journal Of Technology Assessment In Health Care, 24(2), 228–234. https://doi.org/10.1017/S0266462308080318
Mohler, E. R., 3rd., Hiatt, W. R., & Creager, M. A. (2003). Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation, 108(12), 1481–1486. https://doi.org/10.1161/01.CIR.0000090686.57897.F5
Solberg, O. G., Stavem, K., Ragnarsson, A., Beitnes, J. O., Skårdal, R., Seljeflot, I., Ueland, T., Aukrust, P., Gullestad, L., & Aaberge, L. (2019). Index of microvascular resistance to assess the effect of rosuvastatin on microvascular function in women with chest pain and no obstructive coronary artery disease: A double-blind randomized study. Catheterization and Cardiovascular Interventions, 94(5), 660–668. https://doi.org/10.1002/ccd.28157
Strandberg, T. E., Urtamo, A., Kahara, J., Strandberg, A. Y., Pitkala, K. H., & Kautiainen, H. (2018). Statin treatment is associated with a neutral effect on health-related quality of life among community-dwelling octogenarian men: The helsinki businessmen study. The Journals of Gerontology: Series A, 73(10), 1418–1423. https://doi.org/10.1093/gerona/gly073
van Boheemen, L., Tett, S. E., Sohl, E., Hugtenburg, J. G., van Schoor, N. M., & Peeters, G. M. (2016). Associations between statin use and physical function in older adults from the Netherlands and Australia: longitudinal aging study Amsterdam and Australian longitudinal study on women’s health. Drugs Aging., 33(6), 437–445. https://doi.org/10.1007/s40266-016-0370-5
Downs, J. R., Oster, G., & Santanello, N. C. (1993). HMG CoA reductase inhibitors and quality of life. JAMA, 269(24), 3107–3108. https://doi.org/10.1001/jama.1993.03500240051020
Cham, S., Evans, M. A., Denenberg, J. O., & Golomb, B. A. (2010). Statin-associated muscle-related adverse effects: A case series of 354 patients. Pharmacotherapy, 30(6), 541–553. https://doi.org/10.1592/phco.30.6.541
Evans, M. A., & Golomb, B. A. (2009). Statin-associated adverse cognitive effects: Survey results from 171 patients. Pharmacotherapy, 29(7), 541–553. https://doi.org/10.1592/phco.29.7.800
Bhardwaj, S., Selvarajah, S., & Schneider, E. B. (2013). Muscular effects of statins in the elderly female: A review. Clinical Interventions in Aging, 8, 47–59. https://doi.org/10.2147/CIA.S29686
Phillips, P. S., Haas, R. H., Bannykh, S., Hathaway, S., Gray, N. L., Kimura, B. J., Vladutiu, G. D., & England, J. D. F. (2002). Statin-associated myopathy with normal creatine kinase levels. Annals of Internal Medicine, 137(7), 581–585. https://doi.org/10.7326/0003-4819-137-7-200210010-00009
Leplège, A., Ecosse, E., Coste, J., Pouchot, J., & Perneger, T. (2001). Le questionnaire MOS SF-36: manuel de l’utilisateur et guide d’interprétation des scores. Éditions ESTEM.
Ware, J. E., & Gandek, B. (1998). Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. Journal of Clinical Epidemiology, 51(11), 903–912. https://doi.org/10.1016/s0895-4356(98)00081-x
Ware, J. E., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30(6), 473–483.
Ware, J. E., Kosinski, M., & Keller, S. D. (1994). SF-36 Physical and Mental Health Summary Scales: A Users’ Manual. The Health Institute.
Riesco, E., Tessier, S., Pérusse, F., Turgeon, S., Tremblay, A., Weisnagel, J., Doré, J., & Mauriège, P. (2010). Impact of walking on eating behaviors and quality of life of premenopausal and early postmenopausal obese women. Menopause, 17(3), 529–538. https://doi.org/10.1097/gme.0b013e3181d12361
Ware, J. E., & Kosinski, M. (2001). Interpreting SF-36 summary health measures: a response. Quality of Life Research, 10(5), 405–413. https://doi.org/10.1023/a:1012588218728. discussion 415-420.
Add-Ins, J. (2014). Full Factorial Repeated Measures ANOVA Add-In. Retrieved 11/11 from https://community.jmp.com/t5/JMP-Add-Ins/Full-Factorial-Repeated-Measures-ANOVA-Add-In/ta-p/23904?trMode=source
Berent, T., Berent, R., Steiner, S., & Sinzinger, H. (2019). Statin-induced muscular side effects at rest and exercise—An anatomical mapping. Atherosclerosis Supplements, 40, 73–78. https://doi.org/10.1016/j.atherosclerosissup.2019.08.026
Finegold, J. A., Manisty, C. H., Goldacre, B., Barron, A. J., & Francis, D. P. (2014). What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. European journal of preventive cardiology, 21(4), 464–474. https://doi.org/10.1177/2047487314525531
Gupta, A., Thompson, D., Whitehouse, A., Collier, T., Dahlof, B., Poulter, N., Collins, R., & Sever, P. (2017). Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian cardiac outcomes trial—lipid-lowering Arm (ASCOT-LLA): A randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. The Lancet, 389(10088), 2473–2481. https://doi.org/10.1016/s0140-6736(17)31075-9
Howard, J. P., Wood, F. A., Finegold, J. A., Nowbar, A. N., Thompson, D. M., Arnold, A. D., Rajkumar, C. A., Connolly, S., Cegla, J., Stride, C., Sever, P., Norton, C., Thom, S. A. M., Shun-Shin, M. J., & Francis, D. P. (2021). Side effect patterns in a crossover trial of statin, placebo, and no treatment. Journal of the American College of Cardiology, 78(12), 1210–1222. https://doi.org/10.1016/j.jacc.2021.07.022
Cruz, A. C., & Pedreira, M. (2020). Patient-and Family-Centered Care and Patient Safety: reflections upon emerging proximity. Revista Brasileira de Enfermagem, 73(6), e20190672. https://doi.org/10.1590/0034-7167-2019-0672
Acknowledgements
The authors wish to thank all participants.
Funding
The study was supported by the Canadian Institutes of Health Research (CIHR grant MOP 114917 to DRJ, JB, and JF).
Author information
Authors and Affiliations
Contributions
DRJ, JF, and JB designed the study. NL and KG provided clinical coordination, CH participated in data collection, CH managed data validation and entry, and PP, CH, and DRJ performed statistical analyses. All participated in data interpretation. PP, under the direction of DRJ and PM, wrote the manuscript. All authors critically reviewed and accepted the final version of the article.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The protocol was approved by the CHU de Québec—Université Laval ethics committee. All patients provided written informed consent, and the study design complied with the principles of the Declaration of Helsinki and was registered in clinicaltrials.gov (NCT01493648).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peyrel, P., Mauriège, P., Frenette, J. et al. Statin withdrawal and health-related quality of life in a primary cardiovascular prevention cohort. Qual Life Res 32, 1943–1954 (2023). https://doi.org/10.1007/s11136-023-03362-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03362-9