Abstract
Purpose
To analyse the Health-Related Quality of Life (HRQoL) at diagnosis of patients with prostate cancer (PCa) according to tumour extension and urinary symptomatology and to explore factors associated with HRQoL.
Methods
408 Controls and 463 PCa cases were included. Eligibility criteria were a new diagnosis of PCa (cases), 40–80 years of age, and residence in the participating hospitals’ coverage area for ≥ 6 months before recruitment. HRQoL was evaluated using the 12-Item Short-Form Health Survey, Mental (MCS) and Physical Component Summaries (PCS), and urinary symptoms with the International Prostate Symptom Score. HRQoL scores for all PCa cases, according to tumour extension and urinary symptoms, were compared with controls. In addition, information about lifestyles and comorbidities was collected and its association with low HRQoL (lower scores) were explored using logistic regression models.
Results
Overall cases had similar PCS score, but lower MCS score than controls. The lowest standardised scores for both PCS and MCS were reached by cases with severe urinary symptoms and a metastatic tumour [mean (SD); PCS: 41.9 (11.5), MCS: 42.3 (10.3)]. Having “below” PCS and MCS scores was associated with the presence of three or more comorbidities in the cases [aOR = 2.86 (1.19–6.84) for PCS and aOR = 3.58 (1.37–9.31) for MCS] and with severe urinary symptomatology [aOR = 4.71 (1.84–12.08) for PCS and aOR = 7.63 (2.70–21.58) for MCS].
Conclusion
The mental dimension of HRQoL at diagnosis of patients with PCa was lower than in controls, especially for cases with severe urinary symptoms and a metastatic tumour. Comorbidities and urinary symptoms were variables associated with the HRQoL of PCa cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain English summary
Prostate cancer cases have increased in recent years, but mortality rates do not present the same trend. On the contrary, there has been an increase in the number of survivors, and quality of life considered a valuable measurement beyond mere survival. Although the impact of treatments on the quality of life of patients with prostate cancer is well known, this issue is not well explored at the moment of the diagnosis. This study analyses the quality of life at diagnosis of prostate cancer patients, comparing this with a control group of the same base population. Moreover, we evaluated the influence of tumour extension and urinary symptoms and explored other potential factors associated with quality of life in these patients. The results showed that patients with prostate cancer had lower quality of life scores than controls, especially for mental health and in cases with severe symptoms and a metastatic tumour. In addition, lower scores in the quality of life of the cases were associated with the presence of comorbidities and urinary symptoms. Considering the variables associated with HRQoL, evaluating urinary symptoms and comorbidities from the moment of diagnosis could be an important aspect to include in the clinical decision making.
Introduction
Prostate cancer (PCa) poses a significant public health burden and is a major cause of morbidity and mortality worldwide, being the most common cancer in men in most world regions, excluding non-melanoma skin cancers [1, 2]. In recent years, there has been an increase in the number of cases worldwide [3]. Nevertheless, mortality rates have decreased [2]. Spain has become one of the European countries with the lowest mortality rates (13.2 deaths per 100,000 person-years) [4], and the survival rates indicate that approximately 89% of Spanish patients are still alive 5 years after a PCa diagnosis [5].
A consequence of these epidemiological trends of PCa is the growing interest in Health-Related Quality of Life (HRQoL) as a valuable measurement beyond mere survival [6]. The impact of treatments on HRQoL has been widely studied [7]. In this sense, some studies have reported how the different treatments were associated with distinct patterns of adverse effects [8,9,10,11]. Recently, it has been suggested that HRQoL should be integrated into the treatment decision-making process [12, 13]. However, the HRQoL of PCa patients is not only relevant with regard to treatment. It has been shown that the loss of quality of life during the process of diagnosis is one of the most relevant aspects for PCa patients [14, 15].
Previous studies have analysed the HRQoL of PCa patients at the time of diagnosis [16,17,18,19,20]; however, we must consider: (i) the results regarding HRQoL were not always similar between studies, and some found that the HRQoL score is similar to that of the general population [16, 17] and others better [18, 19]. These discrepancies may be due to differences in the characteristics of PCa cases—such as symptoms, tumour aggressiveness or extension, sociodemographic variables, or lifestyles—; (ii) only 2 studies have used the SF-12, allowing the differentiation between physical and mental quality of life; and (iii) factors related to HRQoL differ between studies, with most focusing on non-modifiable (age, bone metastases, Gleason score) factors or those that are difficult to modify (comorbidities or region of residence) [16,17,18,19,20]. However, to our knowledge, no study has evaluated potentially modifiable factors associated with HRQoL in newly diagnosed patients [16,17,18,19,20]. Moreover, the impact that symptoms have on HRQoL during diagnosis maybe be a crucial aspect for PCa patients [15], which has not been studied until now. Such a study could provide useful information to formulate individualised interventions to improve HRQoL from the moment of diagnosis, as has been suggested previously [21].
Thus, considering the increase in the number of prevalent and incident cases of PCa, the importance of the assessment of HRQoL of patients during the PCa diagnostic process, the inconsistency between previous studies, and the lack of studies that consider urinary symptoms as a factor that could be associated with HRQoL, the aims of this study were (1) to compare HRQoL, using the Short-Form Health Survey version 2 (SF-12 v2), of PCa cases at diagnosis with controls from the same base population, according to tumour extension and urinary symptoms through the International Prostate Symptom Score; and (2) to explore possible factors associated with HRQoL of these patients.
Methods
Study design
The CAPLIFE study is a population-based case–control study whose main objective was to investigate the association between lifestyles and PCa risk. This study was carried out at two main University Hospitals in Granada (Spain): Virgen de las Nieves and Clínico San Cecilio University Hospitals and their catchment areas. Participants were invited to participate from May 2017 to March 2020. The main characteristics of the CAPLIFE study have been described elsewhere [22, 23].
Ethical Approval for this study was provided by the Ethics Committee for Biomedical Research of Andalusia in March 2017. All men included were fully informed regarding the study and signed written informed consent before their voluntary participation. Confidentiality of data was secured, removing personal identifiers in the dataset.
Participants
Criteria for inclusion of cases: (1) new diagnosis of PCa with histological confirmation before receiving treatment (International Classification of Diseases and Related Health Problems 10th Revision [ICD-10]: C61 [24]), (2) 40–80 years of age, and (3) residence in the coverage area of the reference hospitals for 6 months or more prior to recruitment. Controls were selected using the same selection criteria as the cases except for the diagnosis of PCa; therefore, the only difference between cases and controls was the disease (PCa).
Cases were recruited and invited to participate in the urology services of both hospitals through the listings of Pathological Anatomy Service. Controls were chosen at random from the patient lists of general practitioners of 16 primary healthcare centres belonging to the Granada-Metropolitan Health District. For the recruitment of controls, the age at diagnosis of PCa (± 5 years) based on the information obtained from the Granada Cancer Registry was considered. Controls were selected by density sampling over time, which allowed greater comparability of cases and controls. Trained interviewers contacted them to make an appointment for the face-to-face interviews.
Data collection
Information was collected using a structured computerised questionnaire and participants’ medical history.
Health-related quality of life (HRQoL)
The SF-12 v2 was used to evaluate HRQoL during the month before the interview. This validated instrument contains 12 questions with 3- or 5-point Likert scales resulting in 8 dimensions (each dimension was built with 1 or 2 questions): Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Emotional Role, and Mental Health [25]. Additionally, two component summaries are calculated from these eight dimensions: Physical Component Summary (PCS) and Mental Component Summary (MCS). Standards-based scores were obtained following the instructions detailed in the manual of the original version of the questionnaire [26]. For standardisation of the 8 dimensions, we used the means and standard deviation. The weighted dimensions were summed to calculate the summary components (PCS and MCS). Means, standard deviations, and the weights established for the Spanish population were used [27]. Finally, both dimensions and summary components were normalised. Higher scores indicate better quality of life. The Cronbach alpha for SF-12 v2 in our sample was 0.88, which indicates acceptable internal consistency reliability.
Measurement of clinical characteristics
The tumour extension was determined according to the European Association of Urology risk groups for biochemical recurrence: localised, locally advanced, and metastatic PCa [28]. The International Society of Urological Pathology was used to determine low (1–2) and high aggressiveness (3–5) [29, 30].
The original International Prostate Symptom Score [31], validated in the Spanish population, was selected to evaluate urinary symptoms [32]. This was based on the answers to seven questions (incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia) and referred to the month before the interview. Each question is scored from 0 to 5; the total score is obtained by adding the score of the items and ranges from 0 to 35 points (a higher score indicates more severe symptoms). A Cronbach's α for the International Prostate Symptom Score items of 0.86 was found for our sample. Participants were classified as follows: (i) without (0 points), (ii) mild (1–7 points), iii) moderate (8–19 points), and iv) severe urinary symptoms (20–35 points). We added the category “without urinary symptoms” to differentiate between those with no symptoms and those with mild symptoms.
Sociodemographic data and personal history
Information was also collected on sociodemographic data (age, education, marital status, and employment status), Body Mass Index (BMI), first-degree family history of PCa, lifestyle factors (smoking status, sedentary, and Mediterranean diet pattern), and comorbidities.
To evaluate comorbidities, subjects were asked if they had been diagnosed with the following diseases: diabetes mellitus (type 1 or 2), cardiovascular diseases (stroke, angina pectoris, and/or acute myocardial infarction), respiratory diseases (asthma and/or chronic obstructive pulmonary disease), hypertension, mental illnesses (anxiety and/or depression), and other previous cancers. From this, two categories were created following what was done by Porreca A et al. in the Pros-IT CNR study [18]: (i) presence of two or fewer comorbidities, and (ii) presence of three or more comorbidities.
The International Physical Activity Questionnaire Short-Form (IPAQ-SF), validated for the Spanish population [33], was used to collect information about physical activity and sedentariness, referring to the week prior to the interview. The level of sedentariness was measured (h/day) and categorised in terciles based on the control group cut-off points (i) tertile 1 (T1): ≤ 6 h/day); (ii) tertile 2 (T2): > 6 − ≤ 9 h/day; and (iii) tertile 3 (T3) > 9 h/day.
In addition, to determine the adherence to the Mediterranean diet pattern, subjects were provided with a semi-quantitative validated Food Frequency Questionnaire (FFQ) [34]. This questionnaire included 134 foods, specifying the portion size for each food, and referred to the 12 months before the interview. Intakes of less than 800 kcal/day and more than 4000 kcal/day were considered implausible extreme energy intakes [35]. Mediterranean diet pattern adherence was measured by MedDietScore [36]. The total score is obtained by adding the score of the 11 items and ranged from 0 (minimum adherence) to 55 (maximum adherence).
Statistical analysis
In the descriptive analysis, the mean and standard deviation (SD) of the continuous quantitative variables and the distribution of absolute and relative frequencies for the qualitative variables were calculated. Student’s t tests, one-way ANOVA, or Chi-squared test were used according to the nature of the variables.
The HRQoL scores of the cases were compared with scores of the dimensions and components of the SF-12v2 of the control group. For this, both groups were categorised by age groups (40–54, 55–64, 65–74, 75–80 years). The effect size of the differences between PCa cases and controls scores was determined using Cohen's d. It was calculated as the mean difference divided by the standard deviation of the control group (it takes value 10 being normalised scores). An effect size of 0.2, 0.5, and 0.8 was considered as small, medium, or large, respectively [37].
To identify factors associated with HRQoL of cases, they were classified as “the same as or better” or “below” PCS and MCS scores than controls according to age group. Multivariable logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (95% CI). Only variables with p values < 0.20 in the crude analysis were included in these multivariate models.
All statistical tests were two sided and statistical significance was set at p < 0.05. Statistical analyses were performed using the statistical program Stata v.15 (Stata Corp., 2017, College Station, TX, USA). Interactive radar plots showing HRQoL by age group are available online as a web application using R software version 3.6.1 and the packages shiny (v.1.4.0.2) and radarchart (v.0.3.1).
Results
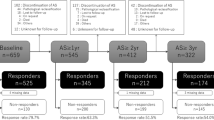
A total of 493 controls and 576 patients with confirmed PCa were invited to participate in the CAPLIFE study. Of these, 430 controls and 470 cases accepted participation in the study. Finally, 408 controls (94.9%) and 463 cases (98.5%) completed the SF-12 v2 (Fig. 1).
Prostate cancer cases and controls characteristics
PCa cases were slightly older, 67.6 years (SD 7.5) vs. 66.0 (SD 7.7), and had a first-degree family history of PCa more frequently (21.9% vs. 10.5%) than the controls. Only 6.7% of cases and 6.6% of the controls had 3 or more comorbidities. Severe urinary symptoms were presented only in 7.1% of the PCa patients, while 27.4% had no urinary symptoms and 38.9% had mild urinary symptoms. According to the tumour extension, metastatic PCa cases were more sedentary, had severe symptoms, and a more aggressive tumour than patients with localised tumours (Table 1).
Scores of physical and mental component summaries
Overall cases had very similar PCS scores but lower MCS scores than controls [53.7 (SD 8.1) vs. 50.8 (SD 9.7)] (Table 2). The most impaired scores were obtained by those cases with a metastatic PCa tumour and severe urinary symptoms [PCS: 41.9 (11.5); MCS: 42.3 (10.3)], with a large effect size for PCS and MCS (0.81 and 1.15, respectively), compared to the controls.
Characteristics of PCa cases categorised as the same/better or below PCS and MCS scores than controls are available in Supplementary Table 1. Overall, 29.4% of PCa had a lower score in PCS, and 52.9% had a lower score in MCS at the moment of diagnosis.
Scores of the eight dimensions of the SF-12 v2
The comparison of the scores for the 8 dimensions of SF-12 v2, for all PCa cases and stratified by tumour extension, and considering the age group is shown in Online Resource 1 (https://dredondo.shinyapps.io/caplife/). Mental Health was impaired in PCa cases with a small–medium effect size for all age ranges. According to the tumour extension, in the group of 65–74 and 75–80 years, metastatic PCa cases had lower scores than localised cases and locally advanced cases in all dimensions. The same occurs in the rest of the age groups (40–54 and 55–64 years), except for the Mental Health dimension. Figure 2 shows only the comparison of the age group with more PCa cases: 65–74 years.
Factors associated with “below” scores of physical and mental component summaries
Table 3 shows the factors associated with having below scores in PCS and MCS compared with the control group. Comorbidities and urinary symptoms were the only variables associated with both PCS and MCS scores. Cases with three or more comorbidities had higher odds of having below scores for both PCS [aOR = 2.86 (95% CI 1.19–6.84)] and MCS [aOR = 3.58 (95% CI 1.37–9.31)]. In the same way, severe urinary symptomology was associated with a below score for PCS [aOR = 4.71 (95% CI 1.84–12.08)] and MCS [aOR = 7.63 (95% CI 2.70–21.58)]. Additionally, there was a significant dose–response relationship between hours a day sitting and having a below PCS score: PCa cases who spent > 6–9 h and ≥ 9 h a day sitting had 2.65 (95% CI 1.48–4.75) and 3.88 (95% CI 2.13–7.06) times more odds of having below PCS score than those who spent ≤ 6 h, respectively. Regarding MCS, every one-unit increase in the score of adherence to a Mediterranean dietary pattern was associated with having the same or better MCS score than controls, with an aOR = 0.94 (95% CI 0.89–0.99). These models explained 14.3% and 7.8% of the variance of PCS and MCS, respectively.
Discussion
Our results indicated that there was a higher percentage of PCa cases with a below MCS score than with a below PCS score compared to the control group and these differences were higher with greater tumour extension and severe urinary symptoms. Regarding the factors associated with poor HRQoL, more severe urinary symptoms and the presence of three or more comorbidities were associated with both component summaries. In addition, sedentariness had a negative impact on PCS, while less adherence to the Mediterranean diet was associated with a below MCS.
In general, PCa cases had very similar PCS scores to controls, consistent with previous studies [18, 19]. However, there was more discrepancy with respect to MCS. We found that PCa patients had more impaired MCS scores than controls. Lane et al. [17] and Harju et al. [19] found similar mental health scores, comparing the patients' HRQoL to UK and Finnish normative data, respectively. These results and the differences with ours may be due to the low aggressiveness of PCa cases analysed in these studies: Lane et al. analysed only localised PCa, and in the study of Harju et al. more than one‐third of the patients initially received non-invasive care. This explanation is supported by the finding that tumour extension had implications for the HRQoL of our patients, with lower scores for locally advanced and metastatic PCa cases, especially for the MCS. Other studies have also found lower scores in HRQoL components in patients with more advanced PCa at diagnosis [16, 18].
In the CAPLIFE study, comorbidity and urinary symptoms were key variables for the HRQoL of PCa patients. The presence of three or more comorbidities was associated with worse HRQoL, both physical and mental. Similar findings have been found in previous studies [18, 19]. In addition, a cohort study observed this effect of comorbidity on PCS from pre-treatment to 36 months [38]. Regarding urinary symptomatology, we added the category “without urinary symptoms” to differentiate between those who had no symptoms and those who had mild symptoms. In our study, those patients with severe symptoms had the most impaired scores, both in PCS and MCS, especially in metastatic cases. Previous studies have suggested paying more attention to identifying and supporting long-term survivors who experience multiple symptoms [39, 40].
Regarding other factors related to HRQoL, we found that being sedentary was associated with below PCS scores and also observed a positive trend between tumour extension and this dimension. Different results have been found in previous studies. Older age, the presence of bone metastases, and higher Gleason score were the factors associated with worse general HRQoL scores in the study of Bergius et al. [16]. This study used a different HRQoL questionnaire, EORTC QLQ-C30, and only included clinical factors in the models. On the other hand, the Pros-IT CNR study [18] found older age, obesity, having comorbidities, and a Gleason score ≥ 8 to be associated with worse PCS score. In this case, the discrepancies could be due to differences in: (1) the included variables: sedentary, first-degree family history of PCa, urinary symptoms, tumour extension, and diet were not evaluated in the Pros-IT CNR study, and (2) the analytical model: we evaluated factors associated with having worse HRQoL score and not the changes in the means of dimensions as done in the Pros-IT CNR study. With our approximation, the model explained 14.5% of the variance in PCS. These results could have practical implications, as with evaluation over time and intervention in modifiable variables such as sedentariness, urinary symptoms, and comorbidity, the PCS scores of PCa cases could approach to those of the control group.
In addition, we also found that less adherence to the Mediterranean diet pattern was related to a below score in MCS. To the best of our knowledge, no previous study has analysed this variable in relation to HRQoL in PCa patients. Considering that previous studies have shown that the decrease in mental health is maintained as the disease evolves [41], the integration of mental care from the beginning, especially in people with other diseases, could help close the gaps found in the follow-up care of PCa survivorships [42].
Some strengths of the present study are: (i) to our knowledge, it is the first study to evaluate the influence of urinary symptoms on HRQoL in newly diagnosed PCa patients. International Prostate Symptom Score was used to evaluate symptoms and SF-12 v2 for HRQoL. Although there are multiple generic and disease-specific HRQoL instruments, none of them has emerged as a gold standard [43]. Specific questionnaires such as Functional Assessment of Cancer Therapy-Prostate (FACT-P), Expanded Prostate Cancer Index Composite (EPIC), or University of California-Los Angeles Prostate Cancer Index (UCLA-PCI) are especially useful for patients already under treatment [44,45,46]. It should be noted that SF-12 v2 has been recommended specifically in PCa patients for its psychometric properties and shortness [47]; (ii) PCS and MCS scores of PCa cases were compared to scores in a control group, belonging to the same area of residence and with very similar sociodemographic characteristics. Previous studies carried out the comparison with a reference population, whose sociodemographic characteristics could differ [19, 48]; (iii) a sensitivity analysis was carried out, comparing the HRQoL scores of our cases not only with the controls but also with the published for Spanish reference population, obtaining similar results (data not shown); (iv) our study included patients with diverse tumour extension, aggressiveness, and urinary symptomatology, which helps to characterise the entire spectrum of the disease; and (v) we evaluated the role of potentially modifiable factors associated with HRQoL such as sedentariness or diet, which can be integrated into the supportive care of PCa patients.
Several limitations should be noted. First, we have not used a disease-specific instrument to evaluate HRQoL. However, this fact should not affect the results as PCa-specific questionnaires aimed to establish the impact of treatments, and our patients were not yet being treated. Second, our results may have been limited by the small sample size and lack of statistical power to detect significant associations. Nevertheless, it was enough to find clinically relevant results, particularly in the effect of urinary symptoms and comorbidity on HRQoL. In addition, although we have analysed several potential factors associated with HRQoL in PCa patients, we cannot rule out the influence of other variables. Finally, the controls were sampled via healthcare centres. In addition to being sampled from centres covering the entire province in urban and rural areas, the participants were randomly selected from the list of family physicians, who care for people with and without pathologies; and controls were possible because the Spanish National Health System has universal access, with a family physician assigned to each user (99.6% of the population of Andalusia is covered by the public health system). The invitation by family physicians facilitated a good participation rate (87.2% in controls; 81.6% in cases).
Conclusions
In conclusion, this study suggests that PCa cases had similar PCS and below MCS scores compared to the control group, and the differences are more pronounced when the cases present severe urinary symptoms and higher tumour extension. Considering the variables associated with the HRQoL of PCa cases, evaluating urinary symptoms and comorbidities from diagnosis could be an important aspect of clinical decision making.
Data availability
Data are available on request due to privacy/ethical restrictions.
Code availability
Not applicable.
References
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D. M., Piñeros, M., Znaor, A., & Bray, F. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 144(8), 1941–1953. https://doi.org/10.1002/ijc.31937
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. https://doi.org/10.3322/caac.21492
Rawla, P. (2019). Epidemiology of prostate cancer. World Journal of Oncology, 10(2), 63–89. https://doi.org/10.14740/wjon1191
Ferlay, J., Colombet, M., Soerjomataram, I., Dyba, T., Randi, G., Bettio, M., Gavin, A., Visser, O., & Bray, F. (2018). Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. European Journal of Cancer, 103, 356–387. https://doi.org/10.1016/j.ejca.2018.07.005
Grosclaude, P., Roche, L., Fuentes-Raspall, R., & Larrañaga, N. (2017). Trends in net survival from prostate cancer in six European Latin countries. European Journal of Cancer Prevention, 26, S114–S120. https://doi.org/10.1097/CEJ.0000000000000304
Bourke, L., Boorjian, S. A., Briganti, A., Klotz, L., Mucci, L., Resnick, M. J., Rosario, D. J., Skolarus, T. A., & Penson, D. F. (2015). Survivorship and improving quality of life in men with prostate cancer. European Urology, 68(3), 374–383. https://doi.org/10.1016/j.eururo.2015.04.023
Lardas, M., Liew, M., van den Bergh, R. C., de Santis, M., Bellmunt, J., van den Broeck, T., Cornford, P., Cumberbatch, M. G., Fossati, N., Gross, T., Henry, A. M., Bolla, M., Briers, E., Joniau, S., Lam, T. B., Mason, M. D., Mottet, N., van der Poel, H. G., Rouvière, O., … Bourke, L. (2017). Quality of life outcomes after primary treatment for clinically localised prostate cancer: A systematic review. European Urology, 72(6), 869–885. https://doi.org/10.1016/j.eururo.2017.06.035
Feyerabend, S., Saad, F., Li, T., Ito, T., Diels, J., Van Sanden, S., De Porre, P., Roiz, J., Abogunrin, S., Koufopoulou, M., & Fizazi, K. (2018). Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: A network meta-analysis. European Journal of Cancer, 103, 78–87. https://doi.org/10.1016/j.ejca.2018.08.010
Alsinnawi, M., Cullen, J., Hurwitz, L. M., Levie, K. E., Burns, J. F., Rosner, I. L., Brand, T. C., Stroup, S., Sterbis, J. R., Rice, K., Conti, G., & Porter, C. R. (2019). A prospective study of health-related quality of life outcomes among men treated for intermediate- and high-risk prostate cancer: The impact of primary and secondary therapies. The Canadian Journal of Urology, 26(4), 9809–9820.
van Stam, M.-A., Aaronson, N. K., Bosch, J. L. H. R., Kieffer, J. M., van der Voort van Zyp, J. R. N., Tillier, C. N., Horenblas, S., & van der Poel, H. G. (2018). Patient-reported outcomes following treatment of localised prostate cancer and their association with regret about treatment choices. European Urology Oncology. https://doi.org/10.1016/j.euo.2018.12.004
Chen, R. C., Basak, R., Meyer, A. M., Kuo, T. M., Carpenter, W. R., Agans, R. P., Broughman, J. R., Reeve, B. B., Nielsen, M. E., Usinger, D. S., Spearman, K. C., Walden, S., Kaleel, D., Anderson, M., Stürmer, T., & Godley, P. A. (2017). Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA—Journal of the American Medical Association, 317(11), 1141–1150. https://doi.org/10.1001/jama.2017.1652
Unger, J. M., Vaidya, R., & Gore, J. L. (2019). Key design and analysis principles for quality of life and patient-reported outcomes in clinical trials. Urologic Oncology: Seminars and Original Investigations, 37(5), 324–330. https://doi.org/10.1016/j.urolonc.2018.02.012
Shevach, J., Weiner, A., & Morgans, A. K. (2019). Quality of life-focused decision-making for prostate cancer. Current Urology Reports, 20(10), 57. https://doi.org/10.1007/s11934-019-0924-2
Msaouel, P., Gralla, R. J., Jones, R. A., & Hollen, P. J. (2017). Key issues affecting quality of life and patient-reported outcomes in prostate cancer: An analysis conducted in 2128 patients with initial psychometric assessment of the prostate cancer symptom scale (PCSS). BMJ Supportive and Palliative Care, 7(3), 308–315. https://doi.org/10.1136/bmjspcare-2016-001146
Sharpley, C. F., Bitsika, V., & Christie, D. R. H. (2018). “The worst thing was…”: Prostate cancer patients’ evaluations of their diagnosis and treatment experiences. American Journal of Men’s Health, 12(5), 1503–1509. https://doi.org/10.1177/1557988318772752
Bergius, S., Torvinen, S., Muhonen, T., Roine, R. P., Taari, K., Bergius, S., Torvinen, S., Muhonen, T., & Roine, R. P. (2016). Health-related quality of life among prostate cancer patients: Real-life situation at the beginning of treatment. Scandinavian Journal of Urology, 51(1), 13–19. https://doi.org/10.1080/21681805.2016.1247293
Lane, A., Metcalfe, C., Young, G. J., Peters, T. J., Blazeby, J., Avery, K. N. L., Dedman, D., Down, L., Mason, M. D., Neal, D. E., Hamdy, F. C., & Donovan, J. L. (2016). Patient-reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: Study design, and baseline urinary, bowel and sexual function and quality of life. BJU International, 118(6), 869–879. https://doi.org/10.1111/bju.13582
Porreca, A., Noale, M., Artibani, W., Bassi, P. F., Bertoni, F., Bracarda, S., Conti, G. N., Corvo, R., Gacci, M., Graziotti, P., Magrini, S. M., Mirone, V., Montironi, R., Muto, G., Pecoraro, S., Ricardi, U., Russi, E., Tubaro, A., Zagonel, V., … Maggi, S. (2018). Disease-specific and general health-related quality of life in newly diagnosed prostate cancer patients: The Pros-IT CNR study. Health and Quality of Life Outcomes, 16(1), 122. https://doi.org/10.1186/s12955-018-0952-5
Harju, E., Rantanen, A., Kaunonen, M., Helminen, M., Isotalo, T., & Astedt-Kurki, P. (2017). The health-related quality of life of patients with prostate cancer and their spouses before treatment compared with the general population. International Journal of Nursing Practice. https://doi.org/10.1111/ijn.12572
Cuypers, M., Lamers, R. E. D., Cornel, E. B., van de Poll-Franse, L. V., de Vries, M., & Kil, P. J. M. (2018). The impact of prostate cancer diagnosis and treatment decision-making on health-related quality of life before treatment onset. Supportive Care in Cancer : Official Journal of the Multinational Association of Supportive Care in Cancer, 26(4), 1297–1304. https://doi.org/10.1007/s00520-017-3953-8
Ralph, N., Ng, S. K., Zajdlewicz, L., Lepore, S. J., Heathcote, P., Kneebone, A., Dunn, J. C., & Chambers, S. K. (2020). Ten-year quality of life outcomes in men with prostate cancer. Psycho-Oncology, 29(2), 444–449. https://doi.org/10.1002/pon.5255
Olmedo-Requena, R., Lozano-Lorca, M., Salcedo-Bellido, I., Jimenez-Pacheco, A., Vazquez-Alonso, F., Garcia-Caballos, M., Sanchez, M.-J., & Jimenez-Moleon, J.-J. (2020). Compliance with the 2018 world cancer research fund/american institute for cancer research cancer prevention recommendations and prostate cancer. Nutrients. https://doi.org/10.3390/nu12030768
Lozano-Lorca, M., Olmedo-Requena, R., Vega-Galindo, M. V., Vázquez-Alonso, F., Jiménez-Pacheco, A., Salcedo-Bellido, I., Sánchez, M. J., & Jiménez-Moleón, J. J. (2020). Night shift work, chronotype, sleep duration, and prostate cancer risk: Caplife study. International Journal of Environmental Research and Public Health, 17(17), 1–17. https://doi.org/10.3390/ijerph17176300
ICD-10 Version:2016. (n.d.). Retrieved January 20, 2020, from https://icd.who.int/browse10/2016/en
Gandek, B., Ware, J. E., Aaronson, N. K., Apolone, G., Bjorner, J. B., Brazier, J. E., Bullinger, M., Kaasa, S., Leplege, A., Prieto, L., & Sullivan, M. (1998). Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. Journal of Clinical Epidemiology, 51(11), 1171–1178. https://doi.org/10.1016/s0895-4356(98)00109-7
Ware, J. E., Jr., Kosinski, M., Turner-Bowker, D. M., Gandek, B. (2002). How to score version 2 of the SF-12® health survey (with a supplement documenting version 1). Lincoln, RI: QualityMetric Incorporated.
Schmidt, S., Vilagut, G., Garin, O., Cunillera, O., Tresserras, R., Brugulat, P., Mompart, A., Medina, A., Ferrer, M., & Alonso, J. (2012). Reference guidelines for the 12-item short-form health survey version 2 based on the catalan general population. Medicina Clinica, 139(14), 613–625. https://doi.org/10.1016/j.medcli.2011.10.024
Mottet, N., Bellmunt, J., Bolla, M., Briers, E., Cumberbatch, M. G., De Santis, M., Fossati, N., Gross, T., Henry, A. M., Joniau, S., Lam, T.B., Mason, M. D., Matveev, V. B., Moldovan, P. C., van den Bergh, R. C. N., Van den Broeck, T., van der Poel, H. G., van der Kwast, T. H., Rouvière, O., Schoots, I. G., Wiegel, T., Cornford, P. (2017). EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol., 71(4), 618–629. https://doi.org/10.1016/j.eururo.2016.08.003.
Neuzillet, Y., Raynaud, J.-P., Dreyfus, J.-F., Radulescu, C., Rouanne, M., Schneider, M., Krish, S., Roupret, M., Drouin, S. J., Comperat, E., Galiano, M., Cathelineau, X., Validire, P., Molinie, V., Fiet, J., Giton, F., Lebret, T., & Botto, H. (2019). Aggressiveness of localized prostate cancer: The key value of testosterone deficiency evaluated by both total and bioavailable testosterone: AndroCan study results. Hormones & Cancer, 10(1), 36–44. https://doi.org/10.1007/s12672-018-0351-8
Epstein, J. I., Egevad, L., Amin, M. B., Delahunt, B., Srigley, J. R., Humphrey, P. A., Grading Committee. (2015). The international society of urological pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. American Journal of Surgical Pathology, 40(2), 244–252. https://doi.org/10.1097/PAS.0000000000000530
Barry, M., Fowler, F. J. J., O’Leary, M. P., Bruskewitz, R. C., Holtgrewe, H. L., Mebust, W. K., & Cockett, A. T. (1992). The American urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. The Journal of Urology, 148(5), 1549–1557. https://doi.org/10.1016/s0022-5347(17)36966-5
Vela Navarrete, R., Martin Moreno, J. M., Calahorra, F. J., Damian Moreno, J., Hernandez Coronado, A., & Boyle, P. (1994). Cultural and linguistic validation, in Spanish, of the international prostatic symptoms scale (I-PSS). Actas urologicas espanolas, 18(8), 841–847.
RománViñas, B., Ribas Barba, L., Ngo, J., & Serra Majem, L. (2013). Validación en población catalana del cuestionario internacional de actividad física. Gaceta Sanitaria, 27(3), 254–257. https://doi.org/10.1016/j.gaceta.2012.05.013
Martin-Moreno, J. M., Boyle, P., Gorgojo, L., Maisonneuve, P., Fernandez-Rodriguez, J. C., Salvini, S., & Willett, W. C. (1993). Development and validation of a food frequency questionnaire in Spain. International Journal of Epidemiology, 22(3), 512–519.
Willett, W. (2013). Nutritional epidemiology. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199754038.001.0001
Panagiotakos, D. B., Pitsavos, C., Arvaniti, F., & Stefanadis, C. (2007). Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Preventive Medicine, 44(4), 335–340. https://doi.org/10.1016/j.ypmed.2006.12.009
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. 2nd edition.
Reeve, B. B., Chen, R. C., Moore, D. T., Deal, A. M., Usinger, D. S., Lyons, J. C., & Talcott, J. A. (2014). Impact of comorbidity on health-related quality of life after prostate cancer treatment: Combined analysis of two prospective cohort studies. BJU International, 114(6b), E74–E81. https://doi.org/10.1111/BJU.12723
Baden, M., Lu, L., Drummond, F. J., Gavin, A., & Sharp, L. (2020). Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Supportive Care in Cancer, 28(10), 4813–4824. https://doi.org/10.1007/s00520-019-05268-0
Bernat, J. K., Wittman, D. A., Hawley, S. T., Hamstra, D. A., Helfand, A. M., Haggstrom, D. A., Darwish-Yassine, M., & Skolarus, T. A. (2016). Symptom burden and information needs in prostate cancer survivors: A case for tailored long-term survivorship care. BJU International, 118(3), 372–378. https://doi.org/10.1111/bju.13329
van Stam, M.-A., van der Poel, H. G., Bosch, J. L. H. R., Tillier, C. N., Horenblas, S., Mols, F., & Aaronson, N. K. (2017). Prevalence and correlates of mental health problems in prostate cancer survivors: A case-control study comparing survivors with general population peers. Urologic Oncology, 35(8), 531.e1-531.e7. https://doi.org/10.1016/j.urolonc.2017.03.028
Crawford-Williams, F., March, S., Goodwin, B. C., Ralph, N., Galvão, D. A., Newton, R. U., Chambers, S. K., & Dunn, J. (2018). Interventions for prostate cancer survivorship: A systematic review of reviews. Psycho-Oncology, 27(10), 2339–2348. https://doi.org/10.1002/pon.4888
Marandino, L., De Luca, E., Zichi, C., Lombardi, P., Reale, M. L., Pignataro, D., Di Stefano, R. F., Ghisoni, E., Mariniello, A., Trevisi, E., Leone, G., Muratori, L., La Salvia, A., Sonetto, C., Buttigliero, C., Tucci, M., Aglietta, M., Novello, S., Scagliotti, G. V., … Di Maio, M. (2019). Quality-of-life assessment and reporting in prostate cancer: Systematic review of phase 3 trials testing anticancer drugs published between 2012 and 2018. Clinical Genitourinary Cancer. https://doi.org/10.1016/j.clgc.2019.07.007
Esper, P., Mo, F., Chodak, G., Sinner, M., Cella, D., & Pienta, K. J. (1997). Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology, 50(6), 920–928. https://doi.org/10.1016/S0090-4295(97)00459-7
Wei, J. T., Dunn, R. L., Litwin, M. S., Sandler, H. M., & Sanda, M. G. (2000). Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology, 56(6), 899–905. https://doi.org/10.1016/s0090-4295(00)00858-x
Litwin, M. S., Hays, R. D., Fink, A., Ganz, P. A., Leake, B., & Brook, R. H. (1998). The UCLA prostate cancer index: Development, reliability, and validity of a health-related quality of life measure. Medical Care, 36(7), 1002–1012. https://doi.org/10.1097/00005650-199807000-00007
Hamoen, E. H. J., De Rooij, M., Witjes, J. A., Barentsz, J. O., & Rovers, M. M. (2015). Measuring health-related quality of life in men with prostate cancer: A systematic review of the most used questionnaires and their validity. Urologic Oncology: Seminars and Original Investigations, 33(2), 69.e19-69.e28. https://doi.org/10.1016/j.urolonc.2013.10.005
Choi, E. P. H., Wong, C. K. H., Tsu, J. H. L., Chin, W. Y., Kung, K., Wong, C. K. W., & Yiu, M. K. (2016). Health-related quality of life of Chinese patients with prostate cancer in comparison to general population and other cancer populations. Supportive Care in Cancer, 24(4), 1849–1856. https://doi.org/10.1007/s00520-015-2980-6
Acknowledgements
We thank all subjects who participated in the study and all CAPLIFE collaborators, and Dr. Ingrid de Ruiter, MBChB PhD, for editorial support in the draft preparation.
Funding
Funding for open access charge: Universidad de Granada / CBUA. This research was funded by Regional Ministry of Health and Families of Andalusia/Consejería de Salud y Familias de la Junta de Andalucía (PI-0514-2016).
Author information
Authors and Affiliations
Contributions
J-JJ-M and RO-R conceived and designed the study. Material preparation, data collection, and analysis were performed by ML-L, RB-R, DR-S, IS-B and RO-R. The coordinated data collection was performed by J-JJ-M, RO-R, J-MC, MA, MG-C, and M-JS. The first draft of the paper was written by ML-L, RB-R, IS-B, and RO-R. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethics Committee of Biomedical Research of Andalusia) in March 2017 and with the Declaration of Helsinki 1964, and its later amendments or comparable ethical standards.
Informed consent
It was obtained from all individual participants in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lozano-Lorca, M., Barrios-Rodríguez, R., Redondo-Sánchez, D. et al. Health-related quality of life in patients newly diagnosed with prostate cancer: CAPLIFE study. Qual Life Res 32, 977–988 (2023). https://doi.org/10.1007/s11136-022-03302-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-022-03302-z