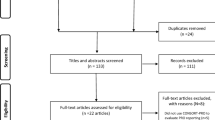
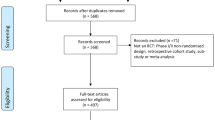
Abstract
Purpose
This study assessed the uptake of the CONsolidated Standards of Reporting Trials (CONSORT)—Patient-Reported Outcomes (PRO) statement; determined if use of CONSORT-PRO was associated with more complete reporting of PRO endpoints in randomised controlled trials (RCTs) and identified the extent to which high-impact journals publishing RCTs with PRO endpoints endorse CONSORT-PRO.
Methods
CONSORT-PRO citations were identified by systematically searching Medline, EMBASE and Google from 2013 (year CONSORT-PRO released) to 17 December 2015. RCTs that cited CONSORT-PRO (cases) were compared to a comparable control sample of RCTs in terms of adherence to CONSORT-PRO using t tests. General linear models assessed the relationship between CONSORT-PRO score and key, pre-specified variables. The 100 highest-impact journals that published RCTs with PRO endpoints (2014–2015) were identified via a systematic Medline search. Instructions for authors were reviewed to determine whether journals endorsed CONSORT-PRO.
Results
Total CONSORT-PRO scores ranged from 47 to 100% for cases and 25–96% for controls. Cases had significantly higher total CONSORT-PRO scores compared to controls: t = 2.64, p = 0.01. ‘Citing CONSORT-PRO’, ‘journal endorsing CONSORT-PRO’ and ‘dedicated PRO paper’ were significant predictors of higher CONSORT-PRO adherence score: R 2 = 0.48, p < 0.001. 11/100 top-ranked journals endorsed CONSORT-PRO in their instructions to authors, seven of these journals published RCTs included as cases in this study.
Conclusion
This study demonstrated improved PRO reporting associated with journal endorsement and author use of the CONSORT-PRO extension. Despite growing awareness, more work is needed to promote appropriate use of CONSORT-PRO to improve completeness of reporting; in particular, stronger journal endorsement of CONSORT-PRO.

Similar content being viewed by others
References
Altman, D., & Simera, I. (2015). A history of the evolution of guidelines for reporting medical research: the long road to the EQUATOR Network. JLL Bulletin: Commentaries on the History of Treatment Evaluation. http://www.jameslindlibrary.org/articles/a-history-of-the-evolution-of-guidelines-for-reporting-medical-research-the-long-road-to-the-equator-network/. Accessed 30 Nov 2015.
Glasziou, P., Altman, D. G., Bossuyt, P., Boutron, I., Clarke, M., Julious, S., et al. (2014). Reducing waste from incomplete or unusable reports of biomedical research. Lancet, 383(9913), 267–276.
Schulz, K. F., Altman, D. G., & Moher, D. (2010). CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials (Vol. 340).
The CONSORT Group Impact of CONSORT. http://www.consort-statement.org/about-consort/impact-of-consort. Accessed 8 Nov 2015.
Turner, L., Shamseer, L., Altman, D. G., Schulz, K. F., & Moher, D. (2012). Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Systematic Reviews, 1(60), 2046–4053. (Review).
Edwards, J. P., Dharampal, N., Chung, W., Brar, M. S., Ball, C. G., Seto, J., et al. (2015). Has the quality of reporting of randomized controlled trials in thoracic surgery improved? European Journal of Cardio-Thoracic Surgery, 49(5):1476–1482. doi:10.1093/ejcts/ezv375. (Journal article)
Lu, J., Gary, K. W., Copolillo, A., Ward, J., Niemeier, J. P., & Lapane, K. L. (2015). Randomized controlled trials in adult traumatic brain injury: a review of compliance to CONSORT statement. Archives of Physical Medicine and Rehabilitation, 96(4), 702–714. doi:10.1016/j.apmr.2014.1010.1026. (Epub 2014 Dec 1019).
Food and Drug Administration (2009). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed 2 Feb 2016.
Calvert, M., Blazeby, J., Altman, D. G., et al. (2013). Reporting of patient-reported outcomes in randomized trials: The CONSORT-PRO extension. JAMA: The Journal of the American Medical Association, 309(8), 814–822. doi:10.1001/jama.2013.879.
Brundage, M., Bass, B., Davidson, J., Queenan, J., Bezjak, A., Ringash, J., et al. (2011). Patterns of reporting health-related quality of life outcomes in randomized clinical trials: implications for clinicians and quality of life researchers. Quality of Life Research, 20(5), 653–664. doi:10.1007/s11136-010-9793-3.
Joly, F., Vardy, J., Pintilie, M., & Tannock, I. F. (2007). Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Annals of Oncology, 18(12), 1935–1942. doi:10.1093/annonc/mdm121.
Calvert, M., Brundage, M., Jacobsen, P. B., Schunemann, H. J., & Efficace, F. (2013). The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health and Quality of Life Outcomes, 11, doi:10.1186/1477-7525-11-184. (Review)
Rothman, K. J., Greenland, S., & Lash, T. (2008). Modern Epidemiology (3rd edn.). Philadelphia, PA: Wolters Kluwer Lippincott Williams & Wilkins.
Enhancing the QUAlity and Transparency Of health Research (EQUATOR Network) (2016). Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. http://www.equator-network.org/reporting-guidelines/consort-pro/. Accessed 11 July 2016.
Babyak, M. A. (2004). What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine, 66(3), 411–421.
Thomson Reuters (2014). Journal Citation Reports Science Edition. http://admin-apps.webofknowledge.com.proxy.queensu.ca/JCR/JCR?PointOfEntry=Home&SID=3C5UmgmjnhOGsy9bXch. Accessed 30 Nov 2015.
Brundage, M., Blazeby, J., Revicki, D., Bass, B., de Vet, H., Duffy, H., et al. (2013). Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Quality of Life Research, 22(6), 1161–1175. doi:10.1007/s11136-012-0252-1.
Vodicka, E., Kim, K., Devine, E. B., Gnanasakthy, A., Scoggins, J. F., & Patrick, D. L. (2015). Inclusion of patient-reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007–2013). Contemporary Clinical Trials, 43, 1–9. doi:10.1016/j.cct.2015.1004.1004.
Bylicki, O., Gan, H. K., Joly, F., Maillet, D., You, B., & Péron, J. (2014). Poor patient-reported outcomes reporting according to CONSORT guidelines in randomized clinical trials evaluating systemic cancer therapy. Annals of Oncology, 26(1), 231–237. doi:10.1093/annonc/mdu489.
Dirven, L., Taphoorn, M. J. B., Reijneveld, J. C., Blazeby, J., Jacobs, M., Pusic, A., et al. (2014). The level of patient-reported outcome reporting in randomised controlled trials of brain tumour patients: A systematic review. European Journal of Cancer, 50(14), 2432–2448. doi:10.1016/j.ejca.2014.06.016.
Efficace, F., Feuerstein, M., Fayers, P., Cafaro, V., Eastham, J., Pusic, A., et al. (2013). Patient-reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making. European Urology, 30(13), 01090–01097. (Journal article)
Efficace, F., Jacobs, M., Pusic, A., Greimel, E., Piciocchi, A., Kieffer, J. M., et al. (2014). Patient-reported outcomes in randomised controlled trials of gynaecological cancers: Investigating methodological quality and impact on clinical decision-making. European Journal of Cancer, 50(11), 1925–1941. doi:10.1016/j.ejca.2014.04.005.
Mercieca-Bebber, R. L., Perreca, A., King, M., Macann, A., Whale, K., Soldati, S., et al. (2016). Patient-reported outcomes in head and neck and thyroid cancer randomised controlled trials: A systematic review of completeness of reporting and impact on interpretation. European Journal of Cancer, 56, 144–161. doi:10.1016/j.ejca.2015.12.025.
Efficace, F., Fayers, P., Pusic, A., Cemal, Y., Yanagawa, J., Jacobs, M., et al. (2015). Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: A pooled analysis of 557 trials. Cancer, 121(18), 3335–3342. doi:10.1002/cncr.29489.
Koller, M., Warncke, S., Hjermstad, M. J., Arraras, J., Pompili, C., Harle, A., et al. (2015). Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer, 121(24), 4300–4323. doi:10.1002/cncr.29682.
Weingartner, V., Dargatz, N., Weber, C., Mueller, D., Stock, S., Voltz, R., et al. (2016). Patient reported outcomes in randomized controlled cancer trials in advanced disease: a structured literature review. Expert Review of Clinical Pharmacology, 9(6), 821–829. doi:10.1586/17512433.17512016.11164595.
Hartling, L., Ospina, M., Liang, Y., Dryden, D. M., Hooton, N., Krebs Seida, J., et al. (2009). Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ, 339. doi:10.1136/bmj.b4012
Mercieca-Bebber, R., Palmer, M. J., Brundage, M., Calvert, M., Stockler, M. R., & King, M. T. (2016). Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open, 6(6). doi:10.1136/bmjopen-2015-010938.
Rouette, J., Blazeby, J., King, M., Calvert, M., Peng, Y., Meyer, R. M., et al. (2015). Integrating health-related quality of life findings from randomized clinical trials into practice: an international study of oncologists’ perspectives. Quality of Life Research, 24(6), 1317–1325. doi:10.1007/s11136-014-0871-9.
CONsolidated Standards of Reporting Trials (CONSORT) Transparent reporting of trials (2013). Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions?ContentWidgetId=560. Accessed 11 July 2016.
UK National Institute for Health Research (NIHR) Research Design Service Resource Resources. http://www.rds-sc.nihr.ac.uk/resources-and-links/. Accessed 11 July 2016.
Acknowledgements
This paper was reviewed and endorsed by the International Society for Quality of Life Research (ISOQOL) Board of Directors as an ISOQOL publication and does not reflect an endorsement of the ISOQOL membership.
We would like to acknowledge members of the ISOQOL Best Practices for PROs—Reporting Taskforce who were not directly involved in this study: Dagmar Amtmann, Claire Burbridge, Sandra Nolte, Claire Snyder, Galina Velikova, Tracey Young and Carole White. RMB is supported by Sydney Catalyst, courtesy of the Cancer Institute New South Wales. MK is supported by the Australian Government, courtesy of Cancer Australia.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Co-authors Calvert, Brundage and King were involved in the original development of CONSORT-PRO, however received no direct benefit from the findings reported here. Prof. Calvert has received grant funding from Macmillan, NIHR, Health Foundation and consultancy payments from Astellas Pharma, and Ferring Pharma, all of which are outside the submitted work. The potential conflicts have not had an impact on the design, conduct, or reporting of the submitted work. This project did not receive any funding.
Ethics statement
This article is an analysis of PRO reporting of RCTs and of journals’ instructions to authors. It did not involve direct study of human participants, and therefore, human research ethics approval was not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mercieca-Bebber, R., Rouette, J., Calvert, M. et al. Preliminary evidence on the uptake, use and benefits of the CONSORT-PRO extension. Qual Life Res 26, 1427–1437 (2017). https://doi.org/10.1007/s11136-017-1508-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1508-6