Abstract
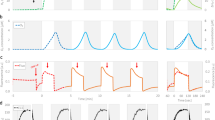
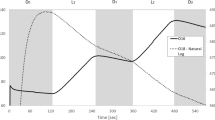
The green algal genus Picochlorum is of biotechnological interest because of its robust response to multiple environmental stresses. We compared the metabolic performance of P. SE3 and P. oklahomense to diverse microbial phototrophs and observed exceptional performance of photosystem II (PSII) in light energy conversion in both Picochlorum species. The quantum yield (QY) for O2 evolution is the highest of any phototroph yet observed, 32% (20%) by P. SE3 (P. okl) when normalized to total PSII subunit PsbA (D1) protein, and 80% (75%) normalized per active PSII, respectively. Three factors contribute: (1) an efficient water oxidizing complex (WOC) with the fewest photochemical misses of any organism; (2) faster reoxidation of reduced (PQH2)B in P. SE3 than in P. okl. (period-2 Fourier amplitude); and (3) rapid reoxidation of the plastoquinol pool by downstream electron carriers (Cyt b6f/PETC) that regenerates PQ faster in P. SE3. This performance gain is achieved without significant residue changes around the QB site and thus points to a pull mechanism involving faster PQH2 reoxidation by Cyt b6f/PETC that offsets charge recombination. This high flux in P. SE3 may be explained by genomically encoded plastoquinol terminal oxidases 1 and 2, whereas P. oklahomense has neither. Our results suggest two distinct types of PSII centers exist, one specializing in linear electron flow and the other in PSII-cyclic electron flow. Several amino acids within D1 differ from those in the low-light-descended D1 sequences conserved in Viridiplantae, and more closely match those in cyanobacterial high-light D1 isoforms, including changes near tyrosine Yz and a water/proton channel near the WOC. These residue changes may contribute to the exceptional performance of Picochlorum at high-light intensities by increasing the water oxidation efficiency and the electron/proton flux through the PSII acceptors (QAQB).






Similar content being viewed by others
Data availability
All data used to generate the figures in this paper and its supporting information are available from C.G., F.F., and G.C.D. on request.
References
Ananyev G, Dismukes GC (2005) How fast can photosystem II split water? Kinetic performance at high and low frequencies. Photosynth Res 84(1–3):355–365. https://doi.org/10.1007/s11120-004-7081-1
Ananyev G, Gates C, Dismukes GC (2016) The Oxygen quantum yield in diverse algae and cyanobacteria is controlled by partitioning of flux between linear and cyclic electron flow within photosystem II. Biochem Biophys Acta 1857:1380–1391
Ananyev G, Gates C, Kaplan A, Dismukes GC (2017) Photosystem II-cyclic electron flow powers exceptional photoprotection and record growth in the microalga Chlorella ohadii. Biochimica et Biophysica Acta (BBA)–Bioenergetics 1858(11):873–883. https://doi.org/10.1016/j.bbabio.2017.07.001
Ananyev G, Roy-Chowdhury S, Gates C, Fromme P, Dismukes GC (2019) The catalytic cycle of water oxidation in crystallized photosystem II complexes: performance and requirements for formation of intermediates. ACS Catal 9(2):1396–1407. https://doi.org/10.1021/acscatal.8b04513
Atlas GS (2019) 2.0, a free, web-based application is developed and operated by the company solargis sro on behalf of the World Bank Group, utilizing Solargis data, with funding provided by the Energy Sector Management Assistance Program (ESMAP). For additional information: https://www.globalsolaratlas.info
Babcock GT, Barry BA, Debus RJ, Hoganson CW, Atamian M, McIntosh L, Sithole I, Yocum CF (1989) Water oxidation in photosystem II: from radical chemistry to multielectron chemistry. Biochemistry 28(25):9557–9565. https://doi.org/10.1021/bi00451a001
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Behrenfeld MJ, Prasil O, Kolber ZS, Babin M, Falkowski PG (1998) Compensatory changes in photosystem II electron turnover rates protect photosynthesis from photoinhibition. Photosynth Res 58(3):259–268
Borowitzka M (2018) Commercial-scale production of microalgae for bioproducts. In: Blue biotechnology, pp 33–65. https://doi.org/10.1002/9783527801718.ch2
Bruce D, Vasil’ev S (2004) Excess light stress: multiple dissipative processes of excess excitation. In: Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Berlin, pp 497–523
Cady CW, Crabtree RH, Brudvig GW (2008) Functional models for the oxygen-evolving complex of photosystem II. Coord Chem Rev 252(3–4):444–455
Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido Gamarro E, Geehan J, Hurtado A (2021) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fish Aquac Circular (1229)
Causmaecker SD, Douglass JS, Fantuzzi A, Nitschke W, Rutherford AW (2019) Energetics of the exchangeable quinone, QB, in Photosystem II. Proc Natl Acad Sci 116(39):19458–19463. https://doi.org/10.1073/pnas.1910675116
Chen LZ, Li DH, Song LR, Hu CX, Wang GH, Liu YD (2006) Effects of salt stress on carbohydrate metabolism in desert soil alga Microcoleus vaginatus Gom. J Integr Plant Biol 48(8):914–919
Council NR (2013) Sustainable development of algal biofuels in the United States. National Academies Press
da Roza PA, Goold HD, Paulsen IT (2022) Picochlorum sp. SENEW3. Trends Genetics 38(2):209–210
de Wijn R, Van Gorkom HJ (2001) Kinetics of electron transfer from QA to QB in photosystem II. Biochemistry 40(39):11912–11922
Demmig-Adams B, Garab G, Adams W III, Govindjee U (2014) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria, vol 40. Springer
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797
Elgin B, Crowley K (2023) Exxon retreats from major climate effort to make biofuels from algae. Bloomberg News
Finazzi G, Forti G (2004) Metabolic flexibility of the green alga Chlamydomonas reinhardtii as revealed by the link between state transitions and cyclic electron flow. Photosynth Res 82:327–338
Foflonker F, Ananyev G, Qiu H, Morrison A, Palenik B, Dismukes GC, Bhattacharya D (2016) The unexpected extremophile: tolerance to fluctuating salinity in the green alga Picochlorum. Algal Res 16:465–472. https://doi.org/10.1016/j.algal.2016.04.003
Foflonker F, Mollegard D, Ong M, Yoon HS, Bhattacharya D (2018) Genomic analysis of picochlorum species reveals how microalgae may adapt to variable environments. Mol Biol Evol 35(11):2702–2711. https://doi.org/10.1093/molbev/msy167
Foflonker F, Price DC, Qiu H, Palenik B, Wang S, Bhattacharya D (2015) Genome of the halotolerant green alga Picochlorum sp. reveals strategies for thriving under fluctuating environmental conditions. Environ Microbiol 17(2):412–426
Gates C, Ananyev G, Dismukes GC (2016) The strontium inorganic mutant of the water oxidizing center (CaMn4O5) of PSII improves WOC efficiency but slows electron flux through the terminal acceptors. Biochimica et Biophysica Acta (BBA)–Bioenergetics 1857(9):1550–1560. https://doi.org/10.1016/j.bbabio.2016.06.004
Gates C, Ananyev G, Dismukes GC (2020) Realtime kinetics of the light driven steps of photosynthetic water oxidation in living organisms by “stroboscopic” fluorometry. Biochimica et Biophysica Acta (BBA)–Bioenergetics 1861(8):148212. https://doi.org/10.1016/j.bbabio.2020.148212
Gates C, Ananyev G, Roy-Chowdhury S, Fromme P, Dismukes GC (2023a) Regulation of light energy conversion between linear and cyclic electron flow within photosystem II controlled by the plastoquinone/quinol redox poise. Photosynth Res 156(1):113–128. https://doi.org/10.1007/s11120-022-00985-w
Gates C, Williams JM, Ananyev G, Dismukes GC (2023b) How chloride functions to enable proton conduction in photosynthetic water oxidation: Time-resolved kinetics of intermediates (S-states) in vivo and bromide substitution. Biochimica et Biophysica Acta (BBA)-Bioenergetics 148998
Gorbunov MY, Kolber ZS, Falkowski PG (1999) Measuring photosynthetic parameters in individual algal cells by fast repetition rate fluorometry. Photosynth Res 62(2):141–153. https://doi.org/10.1023/a:1006360005033
Gouy M, Tannier E, Comte N, Parsons DP (2021) Seaview version 5: a multiplatform software for multiple sequence alignment, molecular phylogenetic analyses, and tree reconciliation. In Multiple sequence alignment: methods and protocols, pp 241–260
Greene GMP, Joseph L. (2023) USDA NASS Acreage Report 2023. National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA), Washington, D.C.
Guerra LT, Xu Y, Bennette N, McNeely K, Bryant DA, Dismukes GC (2013) Natural osmolytes are much less effective substrates than glycogen for catabolic energy production in the marine cyanobacterium Synechococcus sp. strain PCC 7002. J Biotechnol 166(3):65–75
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. Culture of marine invertebrate animals: proceedings—1st conference on culture of marine invertebrate animals greenport. Springer, pp 29–60
Henley WJ, Hironaka JL, Guillou L, Buchheim MA, Buchheim JA, Fawley MW, Fawley KP (2004) Phylogenetic analysis of the ‘Nannochloris-like’algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov.(Trebouxiophyceae, Chlorophyta). Phycologia 43(6):641–652
Hillier W, Babcock GT (2001) S-state dependent Fourier transform infrared difference spectra for the photosystem II oxygen evolving complex. Biochemistry 40(6):1503–1509
Huesemann MH, Knoshaug EP, Laurens LM, Dale T, Lane TW, McGowen J (2022) Development of integrated screening, cultivar optimization, and verification research (DISCOVR): A coordinated research-driven approach to improve microalgal productivity, composition, and culture stability for commercially viable biofuels production. Algal Research 102961
Huntley ME, Johnson ZI, Brown SL, Sills DL, Gerber L, Archibald I, Machesky SC, Granados J, Beal C, Greene CH (2015) Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Res 10:249–265
Hussein R, Ibrahim M, Bhowmick A, Simon PS, Chatterjee R, Lassalle L, Doyle M, Bogacz I, Kim I-S, Cheah MH, Gul S, de Lichtenberg C, Chernev P, Pham CC, Young ID, Carbajo S, Fuller FD, Alonso-Mori R, Batyuk A, Sutherlin KD, Brewster AS, Bolotovsky R, Mendez D, Holton JM, Moriarty NW, Adams PD, Bergmann U, Sauter NK, Dobbek H, Messinger J, Zouni A, Kern J, Yachandra VK, Yano J (2021) Structural dynamics in the water and proton channels of photosystem II during the S2 to S3 transition. Nat Commun 12(1):6531. https://doi.org/10.1038/s41467-021-26781-z
Koivuniemi A, Aro E-M, Andersson B (1995) Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 34(49):16022–16029. https://doi.org/10.1021/bi00049a016
Kumar V, Sharma N, Jaiswal KK, Vlaskin MS, Nanda M, Tripathi MK, Kumar S (2021) Microalgae with a truncated light-harvesting antenna to maximize photosynthetic efficiency and biomass productivity: Recent advances and current challenges. Process Biochem 104:83–91. https://doi.org/10.1016/j.procbio.2021.03.006
Lewis K, Rumpang E, Kho LK, McCalmont J, Teh YA, Gallego-Sala A, Hill TC (2020) An assessment of oil palm plantation aboveground biomass stocks on tropical peat using destructive and non-destructive methods. Sci Rep 10(1):2230
Liu X, Saydah B, Eranki P, Colosi LM, Greg Mitchell B, Rhodes J, Clarens AF (2013) Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Biores Technol 148:163–171. https://doi.org/10.1016/j.biortech.2013.08.112
Mani K, Zournas A, Dismukes GC (2022) Bridging the gap between Kok-type and kinetic models of photosynthetic electron transport within Photosystem II. Photosynth Res 151(1):83–102. https://doi.org/10.1007/s11120-021-00868-6
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177(4):272–280. https://doi.org/10.1016/j.plantsci.2009.06.005
Messinger J, Noguchi T, Yano J (2011) Photosynthetic O2 evolution. In: Wydrzynski TJ, Hillier W (eds) Molecular solar fuels. RSC energy and environment series, vol 5. pp 163–207
Messinger J, Schroeder WP, Renger G (1993) Structure-function relations in photosystem II. Effects of temperature and chaotropic agents on the period four oscillation of flash-induced oxygen evolution. Biochemistry 32(30):7658–7668. https://doi.org/10.1021/bi00081a009
Mulo P, Sicora C, Aro E-M (2009) Cyanobacterial psbA gene family: optimization of oxygenic photosynthesis. Cell Mol Life Sci 66:3697–3710
Nagarajan A, Burnap RL (2012) Patterns of conservation and divergence of the photosystem II complex. In: Functional genomics and evolution of photosynthetic systems, pp 317–344
Nakajima Y, Ugai-Amo N, Tone N, Nakagawa A, Iwai M, Ikeuchi M, Sugiura M, Suga M, Shen J-R (2022) Crystal structures of photosystem II from a cyanobacterium expressing psbA2 in comparison to psbA3 reveal differences in the D1 subunit. J Biol Chem 298(12)
Narala RR, Garg S, Sharma KK, Thomas-Hall SR, Deme M, Li Y, Schenk PM (2016) Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front Energy Res 4:29
Nedbal L, Trtilek M, Kaftan D (1999) Flash fluorescence induction: a novel method to study regulation of photosystem II. J Photochem Photobiol, B 48(2–3):154–157
Negi S, Perrine Z, Friedland N, Kumar A, Tokutsu R, Minagawa J, Berg H, Barry AN, Govindjee G, Sayre R (2020) Light regulation of light-harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae†. Plant J 103(2):584–603. https://doi.org/10.1111/tpj.14751
Pang M, Liu K, Liu H (2022) Evidence for mixotrophy in pico-chlorophytes from a new Picochlorum (Trebouxiophyceae) strain. J Phycol 58(1):80–91
Patil S, Prakash G, Lali AM (2020) Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J Appl Phycol 32(3):1559–1567. https://doi.org/10.1007/s10811-020-02081-9
Perrine Z, Negi S, Sayre RT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1(2):134–142
Pospíšil P (2016) Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci 7:1950
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic Engineering of algae for enhanced biofuel production. Eukaryot Cell 9(4):486–501. https://doi.org/10.1128/EC.00364-09
Ritchie H, Roser M (2021) Forests and deforestation. Our world in data
Robinson HH, Crofts AR (1983) Kinetics of the oxidation—reduction reactions of the photosystem II quinone acceptor complex, and the pathway for deactivation. FEBS Lett 153(1):221–226
Rochaix J-D (2011) Regulation of photosynthetic electron transport. BBA-Bioenergetics 1807(3):375–383. https://doi.org/10.1016/j.bbabio.2010.11.010
Rutherford A, Renger G, Koike H, Inoue Y (1984) Thermoluminescence as a probe of photosystem II. The redox and protonation states of the secondary acceptor quinone and the O2-evolving enzyme. Biochimica et Biophysica Acta (BBA)-Bioenergetics 767(3):548–556
Salguero-Rodríguez Y, Gómez-Pérez C, Arango-Restrepo JP, Espinosa J (2021) Static mixer proposal for tubular photobioreactors to reduce mixing energy consumption and enhance light–dark cycles. J Chem Technol Biotechnol 96(1):113–124
Sartory D, Grobbelaar J (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177–187
Savage DF (2007) Towards membrane protein structure determination. University of California, San Francisco
Scully MJ, Norris GA, Falconi TMA, MacIntosh DL (2021) Carbon intensity of corn ethanol in the United States: state of the science. Environ Res Lett 16(4):043001
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae. NREL, Online
Shetty P, Gitau MM, Maróti G (2019) Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae. Cells 8(12):1657
Stephens TG, Gabr A, Calatrava V, Grossman AR, Bhattacharya D (2021) Why is primary endosymbiosis so rare? New Phytologist
Sugiura M, Boussac A (2014) Some photosystem II properties depending on the D1 protein variants in Thermosynechococcus elongatus. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1837(9):1427–1434
Sugiura M, Ogami S, Kusumi M, Un S, Rappaport F, Boussac A (2012) Environment of TyrZ in Photosystem II from Thermosynechococcus elongatus in which PsbA2 is the D1 protein. J Biol Chem 287(16):13336–13347
Svensson B, Vass I, Styring S (1991) Sequence analysis of the D1 and D2 reaction center proteins of photosystem II. Zeitschrift Für Naturforschung C 46(9–10):765–776
Valin H, Peters D, Van den Berg M, Frank S, Havlik P, Forsell N, Hamelinck C, Pirker J, Mosnier A, Balkovic J (2015) The land use change impact of biofuels consumed in the EU: quantification of area and greenhouse gas impacts
Vass I, Styring S, Hundal T, Koivuniemi A, Aro E, Andersson B (1992) Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc Natl Acad Sci 89(4):1408–1412
Vinyard DJ, Gimpel J, Ananyev GM, Cornejo MA, Golden SS, Mayfield SP, Dismukes GC (2013a) Natural variants of photosystem II subunit D1 tune photochemical fitness to solar intensity. J Biol Chem 288(8):5451–5462. https://doi.org/10.1074/jbc.M112.394668
Vinyard DJ, Gimpel J, Ananyev GM, Mayfield SP, Dismukes GC (2014) Engineered photosystem II reaction centers optimize photochemistry versus photoprotection at different solar intensities. J Am Chem Soc 136(10):4048–4055. https://doi.org/10.1021/ja5002967
Vinyard DJ, Zachary CE, Ananyev G (1827b) Dismukes GC (2013b) Thermodynamically accurate modeling of the catalytic cycle of photosynthetic oxygen evolution: a mathematical solution to asymmetric Markov chains. Biochim Biophys Acta 7:861–868. https://doi.org/10.1016/j.bbabio.2013.04.008
Wang S, Lambert W, Giang S, Goericke R, Palenik B (2014) Microalgal assemblages in a poikilohaline pond. J Phycol 50(2):303–309
Xu X, Sharma P, Shu S, Lin T-S, Ciais P, Tubiello FN, Smith P, Campbell N, Jain AK (2021) Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat Food 2(9):724–732. https://doi.org/10.1038/s43016-021-00358-x
Yang D-H, Andersson B, Aro E-M, Ohad I (2001) The redox state of the plastoquinone pool controls the level of the light-harvesting chlorophyll a/b binding protein complex II (LHC II) during photoacclimation. Photosynth Res 68:163–174
Zarmi Y, Gordon JM, Mahulkar A, Khopkar AR, Patil SD, Banerjee A, Reddy BG, Griffin TP, Sapre A (2020) Enhanced algal photosynthetic photon efficiency by pulsed light. iScience 23(5):101115. https://doi.org/10.1016/j.isci.2020.101115
Zhang Y, Ananyev G, Matsuoka A, Dismukes GC, Maliga P (2023) Cyanobacterial photosystem II reaction center design in tobacco chloroplasts increases biomass in low light. Plant Physiol 191(4):2229–2244
Zournas A, Mani K, Dismukes GC (2023) Cyclic electron flow around photosystem II in silico: how it works and functions in vivo. Photosynth Res 156(1):129–145
Acknowledgements
This work was funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (Grant DE-FG02-10ER16195), and the NIFA-USDA Hatch program (NJ01180). We thank Drs. G.K. Kumaraswamy, Anagha Krishnan, Xiao Qian, and Matt Posewitz for input and advice.
Funding
U.S. Department of Energy, DE-FG02-10ER16195, DE-FG02-10ER16195, National Institute of Food and Agriculture, NJ01180.
Author information
Authors and Affiliations
Contributions
G.C.D. and D.B. conceptualized the manuscript, provided direction, and acquired funding. C.G. and G.A. developed methods. G.A., C.G., and F.F. performed experiments and generated data. C.G., G.A., and F.F. analyzed data. C.G., G.C.D., F.F., and G.A. drafted the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
^This manuscript is dedicated to the memory of Kenneth Sauer, a pioneer in photosynthesis research and a dedicated mentor committed to the personal lives and career aspirations of his students.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gates, C., Ananyev, G., Foflonker, F. et al. Exceptional Quantum Efficiency Powers Biomass Production in Halotolerant Algae Picochlorum sp.^. Photosynth Res (2024). https://doi.org/10.1007/s11120-024-01075-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11120-024-01075-9