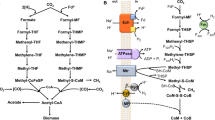
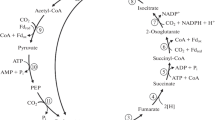
Abstract
For the first decade following its description in 1954, the Calvin–Benson cycle was considered the sole pathway of autotrophic CO2 assimilation. In the early 1960s, experiments with fermentative bacteria uncovered reactions that challenged this concept. Ferredoxin was found to donate electrons directly for the reductive fixation of CO2 into alpha-keto acids via reactions considered irreversible. Thus, pyruvate and alpha-ketoglutarate could be synthesized from CO2, reduced ferredoxin and acetyl-CoA or succinyl-CoA, respectively. This work opened the door to the discovery that reduced ferredoxin could drive the Krebs citric acid cycle in reverse, converting the pathway from its historical role in carbohydrate breakdown to one fixing CO2. Originally uncovered in photosynthetic green sulfur bacteria, the Arnon–Buchanan cycle has since been divorced from light and shown to function in a variety of anaerobic chemoautotrophs. In this retrospective, colleagues who worked on the cycle at its inception in 1966 and those presently working in the field trace its development from a controversial reception to its present-day inclusion in textbooks. This pathway is now well established in major groups of chemoautotrophic bacteria, instead of the Calvin–Benson cycle, and is increasingly referred to as the Arnon–Buchanan cycle. In this retrospective, separate sections have been written by the authors indicated. Bob Buchanan wrote the abstract and the concluding comments.




Similar content being viewed by others
Notes
In this article, the organism currently classified as Chlorobaculum thiosulfatophilum is referred to by its earlier name, Chlorobium thiosulfatophilum.
In writing this article, I have re-examined our original data for the citrate cleavage enzyme in Chlorobium cell extracts. We reported that ATP was added for the formation of 14C-asparate from 14C-isocitrate. Although this was consistent with the operation of the then unknown ATP-citrate lyase, the enzyme was mistakenly referred to as citrate lyase [Table 1, (Evans et al. 1966)]. However, here and in Table 2 of the original publication, the enzyme is referred to as citrate lyase. In writing this article, I have gone back to my laboratory notebook of six decades ago and found that, indeed, ATP was added to the original enzyme assay mixture and, moreover, was required for activity. On the basis of these considerations, I have concluded that the name entered in Tables 1 and 2 of our 1966 paper (citrate lyase) was a misnomer. Had the assays been conducted after 1980 the enzyme should have been designated ATP-citrate lyase (Ivanovsky et al. 1980). Our original assay results are thus consistent with the later observations of these investigators. It is rewarding to see the reconciliation of these data. It was long overdue.
Mike Evans, a co-discoverer of the Arnon–Buchanan citric acid cycle, also obtained his Ph.D. with Elsden.
BBB was pleased to serve as Editor for publication of this interesting article in the Proceedings of the (US) National Academy of Sciences.
References
Aoshima M (2007) Novel enzyme reactions related to the tricarboxylic acid cycle: phylogenetic/functional implications and biotechnological applications. Appl Microbiol Biotechnol 75:249–255
Aoshima M, Ishii M, Igarashi Y (2003) A novel biotin protein required for reductive carboxylation of 2-oxoglutarate by isocitrate dehydrogenase in Hydrogenobacter thermophilus TK-6. Mol Microbiol 51:791–798
Aoshima M, Ishii M, Igarashi Y (2004) A novel enzyme, citryl-CoA synthetase, catalysing the first step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6. Mol Microbiol 52:751–761
Arai H, Kanbe H, Ishii M, Igarashi Y (2010) Complete genome sequence of the thermophilic, obligately chemolithoautotrophic hydrogen oxidizing bacterium Hydrogenobacter thermophilus TK-6. J Bacteriol 192:2651–2652
Ashida H, Saito Y, Kojima C, Kobayashi K, Ogasawara N, Yokota A (2003) A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO. Science 302:286–290
Bachofen R, Buchanan BB, Arnon DI (1964) Ferredoxin as a reductant by a bacterial extract. Proc Natl Acad Sci USA 51:690–694
Berg IA (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77:1925–1936
Berg IA, Keppen OI, Krasil’nikova EN, Ugol’kova NV, Ivanovsky RN (2005) Carbon metabolism of filamentous anoxygenic phototrophic bacteria of the family Oscillochloridaceae. Microbiology (translated from Mikrobiologiya) 74:258–264
Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hügler M, Alber BE, Fuchs G (2010) Autotrophic carbon fixation in archaea. Nat Rev Microbiol 8:447–460
Buchanan BB (2016) The path to thioredoxin in chloroplasts. Annu Rev. Plant Biol 67:1–24
Buchanan BB, Arnon DI (1969) The reductive carboxylic acid cycle. Methods Enzymol 13:170–181
Buchanan BB, Arnon DI (1990) A reverse KREBS cycle in photosynthesis: consensus at last. Photosynth Res 24:47–53
Buchanan BB, Sirevåg R (1976) Ribulose 1,5-diphosphate carboxylase and Chorobium thiosulfatophilum. Arch Microbiol 109:15–19
Bulutoglu B, Garcia KE, Wu F, Minteer SD, Banta S (2016) Direct evidence for metabolon formation and substrate channeling in recombinant TCA cycle enzymes. ACS Chem Biol 11:2847–2853. doi:10.1021/acschembio.6b00523
Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH (1998) Mechanism of Rubisco: the carbamate as general base. Chem Rev 98:549–561
Dalhus B, Saarinen M, Sauer U, Eklund P, Johansson K, Karlsson A, Ramaswamy S, Bjørk A, Synstad B, Naterstad K, Sirevåg R, Eklund H (2002) Structural basis for thermophilic protein stability: structures of thermophilic and mesophilic malate dydrogenases. J Mol Biol 312:707–721
Daniels L, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132:118–126
Dey S, North JA, Sriram J, Evans BS, Tabita FR (2015) In vivo Studies in Rhodospirillum rubrum indicate that ribulose1,5-bisphosphate carboxylase/oxygenase (Rubisco) catalyzes two obligatorily required and physiologically significant reactions for distinct carbon and sulfur metabolic pathways. J Biol Chem 290:30658–30668
Eisen JA, Nelson KE, Paulsen IT, Heidelberg JF, Wu M, Dodson RJ, Deboy R, Gwinn ML, Nelson WC, Haft DH, Hickey EK, Peterson JD, Durkin AS, Kolonay JL, Yang F, Holt I, Umayam LA, Mason T, Brenner M, Shea TP, Parksey D, Nierman WC, Feldblyum TV, Hansen CL, Craven MB, Radune D, Vamathevan J, Khouri H, White O, Gruber TM, Ketchum KA, Venter JC, Tetteli H, Bryant DA, Fraser CM (2002) The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc Natl Acad Sci USA 99:9509–9514
Erb TJ, Evans BS, Cho K, Singh J, Wood BM, Sweedler JV, Tabita FR, Gerlt JA (2012) A RubisCO like protein links SAM metabolism with isoprenoid biosynthesis. Nat Chem Biol 8:926–932
Evans MW, Buchanan BB, Arnon DI (1966) A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci USA 55:928–934
Finn MW, Tabita FR (2003) Synthesis of catalytically active form III ribulose 1,5-bisphosphate carboxylase/oxygenase in archaea. J Bacteriol 185:3049–3059
Fuchs G (ed) (2008) Allgemeine Mikrobiologie, 8th edn. Thieme publisher, Stuttgart
Fuchs G (2011) Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu Rev Microbiol 65:631–658
Fuchs G, Stupperich E, Thauer RK (1978a) Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol 117:61–66
Fuchs G, Stupperich E, Jaenchen R (1978b) Evidence for an incomplete reductive carboxylic acid cycle in Methanobacterium thermoautotrophicum. Arch Microbiol 118:121–125
Fuchs G, Stupperich E, Jaenchen R (1980a) Autotrophic CO2 fixation in Chlorobium limicola. Evidence against the operation of the Calvin cycle in growing cells. Arch Microbiol 128:56–63
Fuchs G, Stupperich E, Eden G (1980b) Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch Microbiol 128:64–71
Gibson J, Tabita FR (1977) Different molecular forms of ribulose 1,5 bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem 252:943 949
Hanson TE, Tabita FR (2001) A RubisCO-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc Natl Acad Sci USA 98:4397–4402
Hanson TE, Tabita FR (2003) Insights into the stress response and sulfur metabolism revealed by proteome analysis of a Chlorobium tepidum mutant lacking the RubisCO-like protein. Photosynth Res 7:231–248
Holo H (1989) Chloroflexus aurantiacus secretes 3-hydroxypropionate, a possible intermediate in the assimilation of CO2 and acetate. Arch Microbiol 13:252–256
Hosoya-Matsuda N, Inoue K, Hisabori T (2009) Roles of thioredoxins in the obligate anaerobic green sulfur bacterium Chlorobium tepidum. Mol Plant 2:336–343
Huber H, Gallenberger M, Jahn U, Eylert E, Berg IA, Kockelkorn D, Eisenreich W, Fuchs G (2008) A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation pathway in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc Natl Acad Sci USA 105:7851–7856
Hügler M, Sievert SM (2011) Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Ann Rev Mar Sci 3:261–289
Ikeda T, Yamamoto M, Arai H et al (2010) Enzymatic and electron paramagnetic resonance studies of anabolic pyruvate synthesis by pyruvate: ferredoxin oxidoreductase from Hydrogenobacter thermophilus. FEBS J 277:501–510
Ishii M, Kawasumi T, Igarashi Y, Kodama T, Minoda Y (1987) 2-Methylthio-1,4-naphthoquinone, a unique sulfur-containing quinone from a thermophilic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. J Bacteriol 169:2380–2384
Ivanovsky RN, Sintsov NV, Kondratieva EN (1980) ATP-linked citrate lyase activity in the green sulfur bacterium Chlorobium limicola forma thiosulfatophilum. Arch Microbiol 128:239–241
Ivanovsky RN, Fal YI, Berg IA, Ugolkova NV, Krasilnikova EN, Keppen OI, Zakharchuc LM, Zyakun AM (1999) Evidence for the presence of the reductive pentose phosphate cycle in a filamentous anoxygenic photosynthetic bacterium, Oscillochloris trichoides strain DG-6. Microbiology 145:1743–1748
Ivanovsky RN, Lebedeva NV, Keppen OI, Tourova TP (2017) Functions of citrate synthase in autotrophic CO2 fixation via the reductive tricarboxylic acid cycle in green sulfur bacteria. Microbiology (Russia) (in press)
Joshi HM, Tabita FR (1996) A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA 93:14515–14520
Kanao TT, Fukui T, Atomi H, Imanaka T (2001) ATP-citrate lyase from the green sulfur bacterium Chlorobium limicola is a heteromeric enzyme composed of two distinct gene products. Eur J Biochem 268:1670–1678
Kawasumi T, Igarashi Y, Kodama T, Minoda Y (1984) Hydrogenobacter thermophilus gen. nov., sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium Int. J Syst Bacteriol 34:5–10
Kim W, Tabita FR (2006) Both subunits of ATP-citrate lyase from Chlorobium tepidum contribute to catalytic activity. J Bacteriol 188:6544–6552
Könneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T, Schada von Borzyskowski L, Erb TJ, Stahl DA, Berg IA (2014) Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc Natl Acad Sci USA 111:8239–8244
Levicán G, Ugalde JA, Ehrenfeld N, Maass A, Parada P (2008) Comparative genomic analysis of carbon and nitrogen assimilation mechanisms in three indigenous bioleaching bacteria: predictions and validations. BMC Genom 9:581
Li H, Sawaya MR, Tabita FR, Eisenberg D (2005) Crystal structure of a novel RuBisCO-like protein from the green sulfur bacterium Chlorobium tepidum. Structure 13:779–789
Ljungdahl LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40:415–450
Lücker S, Wagner M, Maixner F, Pelletier E, Koch H, Vacherie B, Rattei T, Damsté JS, Spieck E, Le Paslier D, Daims H (2010) A nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad Sci USA 107:13479–13484
Madigan MT, Martinko JM, Parker J, Brock TD (1996) Brock biology of microorganisms. Prentice Hall, Upper Saddle River, NJ
Möller D, Schauder R, Fuchs G, Thauer RK (1987) Acetate oxidation to CO2 via a citric acid cycle involving an ATP-citrate lyase: a mechanism for the synthesis of ATP via substrate level phosphorylation in Desulfobacter postgatei growing on acetate and sulfate. Arch Microbiol 148:202–207
Naterstad K, Kolstø AB, Sirevåg R (1995) Physical map of the genome of the green phototrophic bacterium Chlorobium tepidum. J Bacteriol 177:5480–5484
Naterstad K, Lauvrak V, Sirevåg R (1996) Malate dehydrogenase from the mesophile Chlorobium vibriforme and from the mild thermophile Chlorobium tepidum: molecular cloning, construction of a hybrid, and expression in Escherichia coli. J Bacteriol 178:7047–7052
North JA, Sriram J, Chourey K, Ecker CD, Sharma R, Wildenthal JA, Hettich RL, Tabita FR (2016) Metabolic regulation as a consequence of anaerobic 5-methylthioadenosine recycling in Rhodospirillum rubrum. Mbio 7(4):e00855-e00816
Ormerod J (2003) ‘Every dogma has its day’: A personal look at carbon metabolism in photosynthetic bacteria. Photosynth Res 76:135–143
Ramos-Vera WH, Berg IA, Fuchs G (2009) Autotrophic carbon dioxide assimilation in Thermoproteales revisited. J Bacteriol 191:4286–4297
Romagnoli S, Tabita FR (2009) Carbon dioxide metabolism and its regulation in nonsulfur purple photosynthetic bacteria. Advances in photosynthesis respiration, Vol. 28, Springer, Dordrecht
Satagopan S, Chan S, Perry LJ, Tabita FR (2014) Structure-function studies with the unique hexameric form II Rubisco from Rhodopseudomonas palustris. J Biol Chem 289:21433–21450
Schäfer S, Götz M, Eisenreich W, Bacher A, Fuchs G (1989) 13C NMR study of autotrophic CO2 fixation in Thermoproteus neutrophilus. Eur J Biochem 184:151–156
Schauder R, Widdel F, Fuchs G (1987) Carbon assimilation pathways in sulfate-reducing bacteria. II. Enzymes of a reductive citric acid cycle in the autotrophic Desulfobacter hydrogenophilus. Arch Microbiol 148:218–225
Shiba H, Kawasumi T, Igarashi Y, Kodama T, Minoda Y (1985) The CO2 assimilation via the reductive tricarboxylic acid cycle in an obligately autotrophic, aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Arch Microbiol 141:198–203
Singh J, Tabita FR (2010) Roles of RubisCO and the RubisCO-like protein in 5-methylthioadenosine metabolism in the nonsulfur purple bacterium Rhodospirillum rubrum. J Bacteriol 192:1324–1331
Sirevåg R (1975) Photoassimilation of acetate and metabolism of carbohydrate in Chlorbium thiosulfatophilum. Arch Microbiol 104:105–111
Sirevåg R, Castenholz R (1979) Aspects of carbon metabolism in. Chloroflexus. Arch Microbiol 120:151–153
Sirevåg R, Ormerod JG (1970) Carbon dioxide-fixation in photosynthetic green sulfur bacteria. Science 169:186–188
Sirevåg R, Ormerod JG (1977) Synthesis and degradation of polyglucose in Chlorobium thiosulftophilum. Arch Microbiol 111:239–244
Sirevåg R, Buchanan BB, Berry JA, Troughton JH (1977) Mechanisms of CO2 fixation in bacterial photosynthesis studied by the carbon isotope fractionation technique. Arch Microbiol 112:35–38
Srere PA (1959) The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem 234:2544–2547
Strauss G, Fuchs G (1993) Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle. Eur J Biochem 215:633–643
Tabita FR (1999) Microbial ribulose bisphosphate carboxylase/oxygenase: a different perspective. Photosynth Res 60:1–28
Tabita FR, McFadden BA (1974a) One-step isolation of microbial ribulose-1,5-diphosphate carboxylase. Arch Microbiol 99:231–240
Tabita FR, McFadden BA (1974b) d-Ribulose 1,5 diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification of the enzyme and effects of metallic ions on catalysis. J Biol Chem 249:3453–3458
Tabita FR, McFadden BA (1974c) d-Ribulose 1,5 diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic and immunological properties. J Biol Chem 349:3459–3464
Tabita FR, McFadden BA, Pfennig N (1974) D-Ribulose 1,5 diphosphate carboxylase in Chlorobium thiosulfatophilum Tassajara. Biochim Biophys Acta 341:187–194
Tabita FR, Hanson TE, Li H, Satagopan S, Singh J, Chan S (2007) Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs. Microbiol Mol Biol Rev 71:576–599
Tabita FR, Hanson TE, Satagopan S, Witte BH, Kreel NE (2008a) The evolution, structure, and function of RubisCO and its homolog the RubisCO-like protein. Phil Trans R Soc SerB 363:563–576
Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS (2008b) Distinct form I, II, III, and IV. proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J Exp Bot 59:1515–1524
Takeda Y, Suzuki F, Inoue H (1969) ATP citrate lyase (citrate-cleavage enzyme). Methods Enzymol 13:153–160
Tang KH, Blankenship RE (2010) Both forward and reverse TCA cycles operate in green sulfur bacteria. J Biol Chem 285:35848–35854
Thauer RK (2007) A fifth pathway of carbon fixation. Science 318:1732–1733
Thauer RK, Fuchs G, Käufer B, Schnitker U (1974) Carbon monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem 45:343–349
Tourova TP, Spiridonova EM, Slobodova NV, Boulygina ES, Keppen OI, Kuznetsov BB, Ivanovsky RN (2006) Phylogeny of anoxygenic filamentous phototrophic bacteria of the family Oscillochloridaceaea as inferred from comparative analyses of the rrs, cbbL, and nifH genes. Microbiology (translated from Mikrobiologiya) 75:192–200
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci 87:200–204
Wahlund TM, Tabita FR (1997) The reductive tricarboxylic acid cycle of carbon dioxide assimilation: initial studies and purification of ATP-citrate lyase from the green sulfur bacterium Chlorobium tepidum. J Bacteriol 179:4859–4867
Wahlund TM, Woese CR, Castenholz RW, Madigan MT (1991) A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch Microbiol 159:81–90
Warlick BPE, Imker HJ, Sriram J, Tabita FR, Gerlt JA (2012) Mechanistic diversity in the RuBisCO super family: rubisco from Rhodospirillum rubrum is not promiscuous for reactions catalyzed by RubisCO- like proteins. Biochemistry 51:9470–9479
Watson GMF, Yu J-P, Tabita FR (1999) Unusual ribulose 1,5- bisphosphate carboxylase/oxygenase of anoxic archaea. J Bacteriol 181:1569–1575
Wheeldon I, Minteer SD, Banta S, Barton SC, Atanassov P, Sigman M (2016) Substrate channelling as an approach to cascade reactions. Nat Chem 8:299–309
Wrighton KC, Thomas BC, Sharon I, Miller CS, Castelle CJ, VerBerkmoes NC, Wilkins MJ, Hettich RL, Lipton MS, Williams KH, Long PE, Banfield JF (2012) Fermentation, hydrogen and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337:1661–1665
Wrighton KC, Castelle CJ, Varaljay VA, Satagopan S, Brown CT, Wilkins MJ, Thomas BC, Sharon I, Williams KH, Tabita FR, Banfield JF (2016) RubisCO of a pathway known from Archaea is found in diverse uncultivated phyla in bacteria. ISME J 10:2702–2714
Yagi T (1959) Enzymic oxidation of carbon monoxide. J Biochem 46:949–955
Yamamoto M, Ikeda T, Arai H, Ishii M, Igarashi Y (2010) Carboxylation reaction catalyzed by 2-oxoglutarate:ferredoxin oxidoreductases from Hydrogenobacter thermophilus. Extremophiles 14:79–85
Yoon K-S, Hille R, Tabita FR (1999) Rubredoxin from the green sulfur bacterium Chlorobium tepidum functions as an electron acceptor for pyruvate ferredoxin oxidoreductase. J Biol Chem 274:29772–29778
Yoon K-S, Bobst CE, Hemann C, Hille R, Tabita FR (2001) Spectroscopic and functional properties of novel 2[4Fe-4S] cluster-containing ferredoxins from the green sulfur bacterium Chlorobium tepidum. J Biol Chem 276:44027–44036
Zarzycki J, Brecht V, Müller M, Fuchs G (2009) Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci USA 106:21317–21322
Zeikus JG, Fuchs G, Kenealy W, Thauer RK (1977) Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol 132:604–613
Acknowledgements
BBB wishes to acknowledge the kind gift of the wooden dipper by the late Elena Kondratieva (see “Afterword”). Further, on behalf of the authors he thanks Georg Fichs and Ivan Berg for their help in organizing this retrospective. Ivan also did an excellent job in editing the manuscript. Without him the quality of the article would have been compromised. The authors also acknowledge the contributions of Govindjee and Renjie Tang.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was invited by Terry M. Bricker for publication in Photosynthesis Research, and it has been edited by Govindjee.
Afterword
Afterword

Деревянный ковш (derevyannyj kovsh or wooden dipper made of aspen). Memento Elena Kondratieva gave to Bob Buchanan during his 1975 visit to Moscow, Russia, while en route to the 12th International Botanical Congress in Leningrad, U.S.S.R.
Rights and permissions
About this article
Cite this article
Buchanan, B.B., Sirevåg, R., Fuchs, G. et al. The Arnon–Buchanan cycle: a retrospective, 1966–2016. Photosynth Res 134, 117–131 (2017). https://doi.org/10.1007/s11120-017-0429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0429-0