Abstract
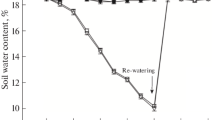
Agave salmiana Otto ex Salm-Dyck, a crassulacean acid metabolism plant that is adapted to water-limited environments, has great potential for bioenergy production. However, drought stress decreases the requirement for light energy, and if the amount of incident light exceeds energy consumption, the photosynthetic apparatus can be injured, thereby limiting plant growth. The objective of this study was to evaluate the effects of drought and re-watering on the photosynthetic efficiency of A. salmiana seedlings. The leaf relative water content and leaf water potential decreased to 39.6 % and −1.1 MPa, respectively, over 115 days of water withholding and recovered after re-watering. Drought caused a direct effect on photosystem II (PSII) photochemistry in light-acclimated leaves, as indicated by a decrease in the photosynthetic electron transport rate. Additionally, down-regulation of photochemical activity occurred mainly through the inactivation of PSII reaction centres and an increased thermal dissipation capacity of the leaves. Prompt fluorescence kinetics also showed a larger pool of terminal electron acceptors in photosystem I (PSI) as well as an increase in some JIP-test parameters compared to controls, reflecting an enhanced efficiency and specific fluxes for electron transport from the plastoquinone pool to the PSI terminal acceptors. All the above parameters showed similar levels after re-watering. These results suggest that the thermal dissipation of excess energy and the increased energy conservation from photons absorbed by PSII to the reduction of PSI end acceptors may be an important acclimation mechanism to protect the photosynthetic apparatus from over-excitation in Agave plants.






Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- ETR:
-
Electron transport rate
- NPQ:
-
Non-photochemical quenching
- OEC:
-
Oxygen evolving complex
- OJIP transient:
-
Fast (seconds) Chl a fluorescence induction curve where O is for the minimum initial fluorescence, J and I are inflections and P is for peak
- PAM:
-
Pulse amplitude modulation
- PAR:
-
Photosynthetic active radiation
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- PQ:
-
Oxidised plastoquinone
- QA :
-
Primary quinone electron acceptor of PSII
- RC:
-
Reaction centre
- SWC:
-
Relative soil water content
- ΨL :
-
Leaf water potential
References
Adamski JM, Peters JA, Daniloski R, Bacarin MA (2011) Excess iron-induced changes in the photosynthetic characteristics of sweet potato. J Plant Physiol 168:2056–2062
Antal TK, Kolacheva A, Maslakov A, Riznichenko GY, Krendeleva TE, Rubin AB (2013) Study of the effect of reducing conditions on the initial chlorophyll fluorescence rise in the green microalgae Chlamydomonas reinhardtii. Photosynth Res 114:143–154
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30:1107–1125
Borland AM, Griffiths H, Hartwell J, Smith AC (2009) Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. J Exp Bot 60:2879–2896
Borland AM, Zambrano VAB, Ceusters J, Shorrock K (2011) The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytol 191:619–633
Broetto F, Duarte HM, Lüttge U (2007) Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3-CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress. J Plant Physiol 164:904–912
Ceppi MG, Oukkaroum A, Çiçek N, Strasser RJ, Schansker G (2012) The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: a study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol Plant 144:277–288
Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP (2009) Differential usage of storage carbohydrates in the CAM bromeliad Aechmea ‘Maya’ during acclimation to drought and recovery from dehydration. Physiol Plant 135:174–184
Chou Y, Polansky AM, Mason RL (1998) Transforming non-normal data to normality in statistical process control. J Qual Technol 30:133–141
Ehleringer J (1981) Leaf absorptances of Mohave and Sonoran desert plants. Oecologia 49:366–370
Flexas J, Barón M, Bota J, Ducruet J-M, Gallé A, Galmés J, Jiménez M, Pou A, Ribas-Carbó M, Sajnani C, Tomàs M, Medrano H (2009) Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandierix, V. rupestris). J Exp Bot 60:2361–2377
Fukuyama K (2004) Structure and function of plant-type ferredoxins. Photosynth Res 81:289–301
Garcia MA (2002) Distribution of Agave (Agavaceae) in Mexico. Cactus Succul J 74:177–187
García-Moya E, Romero-Manzanares A, Nobel PS (2011) Highlights for Agave productivity. GCB Bioenergy 3:4–14
Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218:107–114
Goltsev V, Zaharieva I, Chemev P, Kouzmanova M, Kalaji HM, Yordanov I, Krasteva V, Alexandrov V, Stefanov D, Allakhverdiev SI, Strasser RJ (2012) Drought-induced modifications of photosynthetic electron transport in intact leaves: analysis and use of neural networks as a tool for a rapid non-invasive estimation. BBA-Bioenergetics 1817:1490–1498
Herppich WB, Peckmann K (1997) Responses of gas exchange, photosynthesis, nocturnal acid accumulation and water relations of Aptenia cordifolia to short-term drought and rewatering. J Plant Physiol 150:467–474
Herppich WB, Peckmann K (2000) Influence of drought on mitochondrial activity, photosynthesis, nocturnal acid accumulation and water relations in the CAM plants Prenia sladeniana (ME-type) and Crassula lycopodioides (PEPC-type). Ann Bot 86:611–620
Herppich WB, Midgley GF, Herppich M, Tuffers A, Veste M, von Willert DJ (1998) Interactive effect of photon fluence rates and drought on CAM-cycling in Delosperma tradescantoides (Mesembryanthemaceae). Physiol Plant 102:148–154
Jiang HX, Chen LS, Zheng JG, Han S, Tang N, Smith BR (2008) Aluminum-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol 28:1863–1871
Johnson GN (2011) Physiology of PSI cyclic electron transport in higher plants. BBA-Bioenergetics 1807:384–389
Krüger TPJ, Ilioaia C, Johnson MP, Ruban AV, Papagiannakis E, Horton P, van Grondelle R (2012) Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys J 102:2669–2676
Lazár D (2009) Modelling of light-induced chlorophyll a fluorescence rise (O–J–I–P transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 47:483–498
Lee HSJ, Lüttge U, Medina E, Smith JAC, Cram WJ, Diaz M, Griffiths H, Popp M, Schäfer C, Stimmel K-H, Thonke B (1989) Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in northern Venezuela III. Bromelia humilis Jacq. A terrestrial CAM bromeliad. New Phytol 111:253–271
Lüttge U (1990) Nocturnal citrate accumulation and its response to environmental stress in the CAM plant Kalanchoë pinnata (Lam.) Pers. Plant Cell Environ 13:977–982
Lüttge U (2004) Ecophysiology of crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Makarov SS, Grachev EA, Antal TK (2012) Mathematical modeling of photosynthetic electron transport chain considering spatial heterogeneity of the thylakoid membrane. Math Biol Bioinform 7:508–528
Matiz A, Mioto PT, Mayorga AY, freschi L, Mercier H (2013) CAM photosynthesis in Bromeliads and Agaves: what can we learn from these plants? In: Zvy Dubinsky (ed) Photosynthesis, ISBN: 978-953-51-1161-0, InTech. doi:10.5772/56219. Available from: http://www.intechopen.com/books/photosynthesis/cam-photosynthesis-in-bromeliads-and-agaves-what-can-we-learn-from-these-plants-
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Michalowski CB, Olsen SW, Piepenbrock M, Schmitt JM, Bohnert HJ (1989) Time course of mRNA induction elicited by salt stress in the common ice plant (Mesembryanthemum crystallinum). Plant Physiol 89:811–816
Munday JC Jr, Govindjee (1969) Light-induced changes in the fluorescence yield of chlorophyll a in vivo III. The dip and the peak in the fluorescence transient of Chlorella pyrenoidosa. Biophys J 9:1–21
Munekage Y, Shinakai T (2005) Cyclic electron transport through photosystem I. Plant Biotechnol 22:361–369
Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16:307–314
Nobel PS (1991) Achievable productivities of CAM plants: basis for high values compared with C3 and C4 plants. New Phytol 119:183–205
Nobel PS (1996) High productivity of certain agronomic CAM species. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Springer, Berlin, pp 255–265
Nobel PS (2010) Desert wisdom/agaves and cacti: CO2, water, climate change. iUniverse, New York
Oukarroum A, Madidi SE, Schansker G, Strasser RJ (2007) Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and rewatering. Environ Exp Bot 60:438–446
Oukarroum A, Schansker G, Strasser RJ (2009) Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol Plant 137:188–199
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer (now Springer), Dordrecht
Papageorgiou GC, Govindjee (2011) Photosystem II fluorescence: slow changes—scaling from the past. J Photochem Photobiol B 104:258–270
Patel A, Ting IP (1987) Relationship between respiration and CAM-cycling in Peperomia camptotricha. Plant Physiol 84:640–642
Pieters AJ, Tezara W, Herrera A (2003) Operation of the xanthophyll cycle and degradation of D1 protein in the inducible CAM plant, Talinum triangulare, under water deficit. Ann Bot 92:393–399
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficient and simultaneous equations for assaying chlorophyll a and chlorophyll b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenergetics 975:384–394
Raven JA (2011) The cost of photoinhibition. Physiol Plant 142:87–104
Rayder L, Ting IP (1983) CAM-idling in Hoya carnosa (Asclepiadaceae). Photosynth Res 4:203–211
Redillas MCFR, Strasser RJ, Jeong JS, Kim YS, Kim J-K (2011) The use of JIP test to evaluate drought tolerance of transgenic rice overexpressing OsNAC10. Plant Biotechnol Rep 5:169–175
Rochaix JD (2011) Regulation of photosynthetic electron transport. BBA-Bioenergetics 1807:375–383
Schansker G, Tóth SZ, Strasser RJ (2005) Methyl viologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. BBA-Bioenergetics 1706:250–261
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199–217
Smith JAC, Lüttge U (1985) Day-night changes in leaf water relations associated with the rhythm of crassulacean acid metabolism in Kalanchoë daigremontiana. Planta 163:272–282
Stirbet A (2013) Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynth Res 116:189–214
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Strasser RJ, Stirbet AD (1998) Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O–J–I–P). Math Comput Simul 48:3–9
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer Press, Netherlands, pp 321–362
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. BBA-Bioenergetics 1797:1313–1326
Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149:1560–1567
Tardieu F (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63:25–31
Ting IP (1985) Crassulacean acid metabolism. Annu Rev Plant Physiol 36:595–622
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants’ vitality: applications in detecting and evaluating the impact of mycorrhization on host plants. In: Varma A (ed) Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics, 3rd edn. Springer, Berlin, pp 679–703
van Heerden PDR, Swanepoel JW, Kruger GHJ (2007) Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ Exp Bot 61:124–136
Wilhelm C, Selmar D (2011) Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. J Plant Physiol 168:79–87
Winter K, Garcia M, Holtum JAM (2008) On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë and Opuntia. J Exp Bot 59:1829–1840
Yan K, Chen P, Shao H, Shao C, Zhao S, Brestic M (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS One 8:e62100. doi:10.1371/journal.pone.0062100
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimili-Michael M, Govindjee, Sarin NB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. BBA-Bioenergetics 1797:1428–1438
Acknowledgments
This work was supported by a scholarship (166340) from Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campos, H., Trejo, C., Peña-Valdivia, C.B. et al. Photosynthetic acclimation to drought stress in Agave salmiana Otto ex Salm-Dyck seedlings is largely dependent on thermal dissipation and enhanced electron flux to photosystem I. Photosynth Res 122, 23–39 (2014). https://doi.org/10.1007/s11120-014-0008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0008-6