Abstract
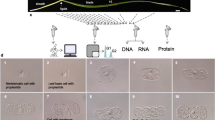
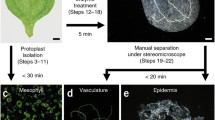
The paraveinal mesophyll (PVM) layer of soybean leaves, which contains cells with various unique ultrastructural properties, has been studied for decades, and several hypotheses regarding its functional role have been developed. Here, we describe a method for obtaining PVM cells using laser capture microdissection and pressure catapulting, subsequent isolation of RNA from these cells, and downstream microarray analysis. A cell type-specific transcriptome analysis was used to compare the gene expression patterns in PVM cells with those of a mesophyll cell type (palisade parenchyma) as a reference. Transcripts related to vegetative storage protein (Vsp) and certain vegetative lipoxygenase (Vlx) isoforms were significantly enriched in PVM cells, which is in accordance with prior work that demonstrated an accumulation of the corresponding proteins. Potential roles of Vsp and Vlx in phosphate mobilization and defense responses, respectively, are discussed. In addition, we found an enrichment of several transport-related genes in PVM cells. Building on our transcriptome data, we provide a fresh discussion of the hypothesis that the PVM plays a role in photoassimilate mobilization and translocation.


Similar content being viewed by others
Abbreviations
- LMPC:
-
Laser microdissection and pressure catapulting
- PAP:
-
Purple acid phosphatase
- PP:
-
Palisade parenchyma
- PVM:
-
Paraveinal mesophyll
- SUF:
-
Sucrose facilitator
- Vlx:
-
Vegetative lipoxygenase
- Vsp:
-
Vegetative storage protein
References
Ayre BG (2011) Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant 4:377–394
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B 57:289–300
Brubaker CL, Lersten NR (1995) Paraveinal mesophyll: review and survey of the subtribe Erythrinae (Phaseolae, Papilionoideae, Leguminosae). Plant Syst and Evol 196:31–62
Bunker TW, Koetje DS, Stephenson LC, Creelman RA, Mullet JE, Grimes HD (1995) Sink limitation induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. Plant Cell 7:1319–1331
Chanroj S, Lu Y, Padmanaban S, Nanatani K, Uozumi N, Rao R, Sze H (2011) Plant-specific cation/H+ exchanger and its homologs are endomembrane K+ transporters with roles in protein sorting. J Biol Chem 286:33931–33941
Chen KM, Holmström M, Raksajit W, Suorsa M, Piippo M, Aro EM (2010) Small chloroplast-targeted DNAJ proteins are involved in optimization of photosynthetic reactions in Arabidopsis thaliana. BMC Plant Biol 10:43
Chi YH, Moon JC, Park JH, Kim HS, Zulfugarow IS, Fanata WI, Jang HH, Lee JR, Lee YM, Kim ST, Chung YY, Lim CO, Kim JY, Yin DJ, Lee CH, Lee KO, Lee SY (2008) Abnormal chloroplast development and growth inhibition in rice thioredoxin m knock-down plants. Plant Physiol 148:808–817
Deeken R, Ache P, Kajahn I, Klinkenberg J, Bringmann G, Hedrich R (2008) Identification of Arabidopsis thaliana phloem RNAs provides a search criterion for phloem-based transcripts hidden in complex datasets of microarray experiments. Plant J 55:746–759
Demchenko K, Zdyb A, Feussner I, Pawlowski K (2012) Analysis of the subcellular localization of lipoxygenase in legume and actinorhizal nodules. Plant Biol 14:56–63
DeWald DB, Mason HS, Mullet JE (1992) The soybean vegetative storage proteins VSPα and VSPβ are acid phosphatases active on phosphates. J Biol Chem 267:15958–15964
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) AgriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38:W64–W70
Dubbs WE, Grimes HD (2000) The mid-pericarp cell layer in soybean pod walls is a multicellular compartment enriched in specific lipoxygenase isoforms. Plant Physiol 123:1281–1288
Everard JD, Franceschi VR, Ku MSB (1990) Characteristics and carbon metabolism of mesophyll and paraveinal mesophyll protoplasts from leaves of non-nodulated Glycine max. Plant Sci 66:167–172
Felton GW (2005) Indigestion in a plant’s best defense. Proc Natl Acad Sci USA 52:18771–18772
Fischer AM, Dubbs WE, Baker RA, Fuller MA, Stephenson LC, Grimes HD (1999) Protein dynamics, activity and cellular localization of soybean lipoxygenases indicate distinct functional roles for individual isoforms. Plant J 19:543–554
Fisher DB (1967) An unusual layer of cells in the mesophyll of the soybean leaf. Bot Gaz 128:215–218
Fisher DB (1970a) Kinetics of C-14 translocation in soybeans: II. Kinetics in the leaf. Plant Physiol 45:114–118
Fisher DB (1970b) Kinetics of C-14 translocation in soybeans: III. Theoretical considerations. Plant Physiol 45:119–125
Franceschi VR, Giaquinta RT (1983a) The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. II. Structural, metabolic, and compartmental changes during reproductive growth. Planta 157:422–431
Franceschi VR, Giaquinta RT (1983b) Specialized cellular arrangements in legume leaves in relation to assimilate transport and compartmentation. Comparison of the paraveinal mesophyll. Planta 159:415–422
Franceschi VR, Wittenbach VA, Giaquinta RT (1983) Paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. III. Immunohistochemical localization of specific glycopeptides in the vacuole after depodding. Plant Physiol 72:586–589
Fuller MA, Weichert H, Fischer AM, Feussner I, Grimes HD (2001) Activity of soybean lipoxygenase isoforms against esterified fatty acids indicates functional specificity. Arch Biochem Biophys 388:146–154
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Geiger D, Becker D, Lacombe B, Hedrich R (2002) Outer pore residues control the H+ and K+ sensitivity of the Arabidopsis potassium channel AKT3. Plant Cell 14:1859–1868
Huang H, Patskovsky Y, Toro R, Farelli JD, Pandya C, Almo SC, Allen KN, Dunaway-Mariano D (2011) Divergence of structure and function in the haloacid dehalogenase enzyme superfamily: Bacteroides thetaiotaomicron BT2127 is an inorganic pyrophosphatase. Biochemistry 50:8937–8949
Itoh R, Fujiwara M, Nagata N, Yoshida S (2001) A chloroplast protein homologous to the eubacterial topological specificity factor MinE plays a role in chloroplast division. Plant Physiol 127:1644–1655
Jauh GY, Fischer AM, Grimes HD, Ryan CA, Rogers JC (1998) δ-Tonoplast intrinsic protein defines unique plant vacuole functions. Proc Natl Acad Sci USA 95:12995–12999
Kato T, Shirano Y, Iwamoto H, Shibata D (1993) Soybean lipoxygenase L-4, a component of the 94-kDa storage protein in vegetative tissues: expression and accumulation in leaves induced by pod removal and by methyl jasmonate. Plant Cell Physiol 34:1063–1072
Klauer SF, Franceschi VR (1997) Mechanism of transport of vegetative storage proteins to the vacuole of the paraveinal mesophyll. Protoplasma 200:174–185
Köpff F (1892) Über die anatomischen Charaktere der Dalbergieen, Sophoreen und Swartzieen, Dissertation, Friedrich-Alexander-Universität, Erlangen, Germany
Lansing AJ, Franceschi VR (2000) The paraveinal mesophyll: a specialized path for intermediary transfer of assimilates in legume leaves. Aust J Plant Physiol 27:757–767
Li C, Gui S, Yang T, Walk T, Wang X, Liao H (2012) Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann Bot 109:275–285
Liu Y, Ahn JE, Datta S, Salzman RA, Moon J, Huyghues-Despointes B, Pittendringh B, Murdock LL, Koiwa H, Zhu-Salzman K (2005) Arabidopsis vegetative storage protein is an anti-insect phosphatase. Plant Physiol 139:1545–1556
Murphy KA, Kuhle RA, Fischer AM, Anterola AM, Grimes HD (2005) The functional status of paraveinal mesophyll vacuoles changes in response to altered metabolic conditions in soybean leaves. Funct Plant Biol 32:335–344
Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM, Sze H (2007) Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol 144:82–93
Sadka A, DeWald DB, May GD, Park WD, Mullet JE (1994) Phosphate modulates transcription of soybean VspB and other sugar-inducible genes. Plant Cell 6:737–749
Saravitz DM, Siedow JN (1996) The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol 110:287–299
Sellhorn GE, Youn B, Webb BN, Gloss LM, Kang C, Grimes H (2011) Biochemical characterization, kinetic analysis and molecular modeling of recombinant vegetative lipoxygenases from soybean. Int J Biol 3:44–62
Smyth GK (2005) Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W (eds) Bioinformatics and computational biology solutions using R and Bioconductor. Springer, New York, pp 397–420
Staswick PE (1989) Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol 89:309–315
Staswick PE (1990) Novel regulation of vegetative storage protein genes. Plant Cell 2:1–6
Staswick PE (1994) Storage proteins of vegetative plant tissues. Annu Rev Plant Physiol Plant Mol Biol 45:303–302
Staswick PE, Zhang Z, Clemente TE, Specht JE (2001) Efficient down-regulation of the major vegetative storage protein genes in transgenic soybean does not compromise plant productivity. Plant Physiol 127:1819–1826
Stephenson LC, Bunker TW, Dubbs WE, Grimes HD (1998) Specific soybean lipoxygenases localize to discrete subcellular compartments and their mRNAs are differentially regulated by source-sink status. Plant Physiol 116:923–933
Szczerba MW, Britto DT, Kronzucker HJ (2009) K+ transport in plants: physiology and molecular biology. J Plant Physiol 166:447–466
Takahashi H, Kamakura H, Sato Y, Shiono K, Abiko T, Tsutsumi N, Nagamura Y, Nishizawa NK, Nakazono M (2010) A method for obtaining high quality RNA from paraffin sections of plant tissues by laser microdissection. J Plant Res 123:807–813
Taylor-Teeples M, Ron M, Brady S (2011) Novel biological insights revealed from cell type-specific expression profiling. Curr Op Plant Biol 14:601–607
Tran HT, Hurley BA, Plaxton WC (2010) Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition. Plant Sci 179:14–27
Tranbarger TJ, Franceschi VR, Hildebrand DF, Grimes HD (1991) The soybean 94-kDa vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3:973–987
Turner GW, Grimes HD, Lange BM (2011) Vegetative lipoxygenases are not storage enzymes. Funct Plant Biol 38:778–787
Turner GW, Cuthbertson DJ, Voo SS, Settles ML, Grimes HD, Lange BM (2012) Experimental sink removal induced stress responses, including shifts in amino acid and phenylpropanoid metabolism, in soybean leaves. Planta 235:939–954
Wedel N, Soll J, Paap BK (1997) CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc Natl Acad Sci USA 94:10479–10484
Wittenbach VA (1983) Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol 73:121–124
Wu Z, Irizarry RA, Gentleman R, Murillo FM, Spencer F (2004) A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99:909–917
Youn B, Sellhorn GE, Mirchel RJ, Gaffney BJ, Grimes HD, Kang C (2006) Crystal structures of vegetative soybean lipoxygenase VLX-B and VLX-D, and comparisons with seed isoforms LOX-1 and LOX-3. Proteins 65:1008–1020
Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW (2007) A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J 49:750–764
Zhu-Salzman K, Luthe DS, Felton GW (2008) Arthropod-inducible proteins: broad spectrum defenses against multiple herbivores. Plant Physiol 146:852–858
Acknowledgments
This work was funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-FG02-09ER16054. The authors would like to thank Mr. Derek Pouchnik (School of Molecular Biosciences, WSU) and Mr. Maximilian Feldman for technical assistance with microarray hybridizations and data analysis. The authors are grateful to Ms. Susan Vogtman for help with growing plants. We would also like to thank Dr. Michael Knoblauch and the staff at WSU’s Franceschi Microscopy and Imaging Center (Drs. Valerie Lynch-Holm and Christine Davitt) for advice on microscopy techniques.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Annotation of genes expressed in paraveinal mesophyll (PVM) cells. The list is based on a comparison of transcript levels between isolated PVM and palisade parenchyma (PP) cells.
ESM 1
(XLS 62 kb)
Rights and permissions
About this article
Cite this article
Voo, S.S., Grimes, H.D. & Lange, B.M. Cell Type-Specific Transcriptome Analysis of the Soybean Leaf Paraveinal Mesophyll Layer. Plant Mol Biol Rep 31, 210–221 (2013). https://doi.org/10.1007/s11105-012-0494-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-012-0494-7