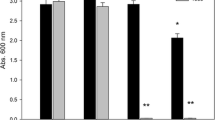
Abstract
Background and aims
Understanding the interactions between endophytic bacteria, rhizobia, free living root associated bacteria and their host plants under stressed conditions remains a significant challenge for proposing strategies to improve the efficacy of PGPR. In this study we analyzed the role of the endophytic bacterium Stenotrophomonas rhizophila in alleviating salinity stress in plants. The nodulation efficiency, plant growth, nitrogen and phosphorus uptake of soybean under hydroponic salt stress conditions were determined.
Methods
Soybean seedlings were inoculated with Bradyrhizobium japonicum BDYD1 and S. rhizophila ep-17 were grown in hydroponic plastic pots containing 2 l of Hoagland solution for 42 days. Salinity conditions were established by adding 50 and 75 mM NaCl to the nutrient solution.
Results
The results showed that the salinity decreased the colonization of B. japonicum BDYD1 in the rhizosphere of soybean, inhibited shoot, root growth, and nodulation compared with those of unstressed plants. We found synergistic interactions between compatible salt tolerant S. rhizophila ep-17 and B. japonicum BDYD1 strains which were manifested themselves as improved root, shoot length, dry weight, N and P uptake and number of nodules compared with the uninoculated plants grown under 75 mM NaCl condition.
Conclusions
S. rhizophila and Bradyrhizobium build beneficial association in the rhizosphere and can act synergistically on promoting plant growth, nutrient uptake and fitness of hydroponically grown soybean under salt stress condition.





Similar content being viewed by others
References
Alavi P, Starcher MR, Zachow C, Müller H, Berg G (2013) Root-microbe systems: the effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405T. Front Plant Sci 4:141
Al-Whaibi MH, Siddiqui MH, Al-Munqadhi BMA, Sakran AM, Ali HM, Basalah MO (2012) Influence of plant growth regulators on growth performance and photosynthetic pigments status of Eruca sativa Mill. J Med Plants Res 6:1948–1954
Argaw A (2012) Evaluation of co-inoculation of Bradyrhizobium japonicum and phosphate solubilizing Pseudomonas spp. effect on soybean (Glycine max L. (Merr.)) in Assossa Area. J Agric Sci Tech 14:213–224
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42(4):2579–2587
Berg G, Martinez JL (2015) Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front Microbiol 6:241
Berg G, Egamberdieva D, Lugtenberg B, Hagemann M (2010) Symbiotic plant-microbe interactions: stress protection, plant growth promotion and biocontrol by Stenotrophomonas. In: Seckbach J, Grube M (eds) Symbioses and stress, cellular origin, life in extreme habitats and astrobiology, Springer-Verlag 17(4):445–460
Berg G, Alavi M, Schmidt CS, Zachow C, Egamberdieva D, Kamilova F, Lugtenberg B (2013) Biocontrol and osmoprotection for plants under saline conditions. In: Frans J. de Bruijn (ed), Molecular microbial ecology of the rhizosphere, Wiley -Blackwell, USA
Beringer JB (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107
Bruning B, Rozema J (2013) Symbiotic nitrogen fixation in legumes: perspectives for saline agriculture. Environ Exp Bot 92:134–143
Dardanelli MS, De Cordoba FJF, Espuny MR, Carvajal MAR, Diaz MES, Serrano AMG, Okon Y, Megias M (2008) Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol Biochem 40:2713–2721
Dwevedi A, Kayastha AM (2011) Soybean: a multifaceted legume with enormous economic capabilities, soybean - biochemistry, chemistry and physiology, Tzi-Bun Ng (Ed.) In Tech
Egamberdieva D (2009) Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Phys Plant 31:861–864
Egamberdieva D (2012) Pseudomonas chlororaphis: a salt tolerant bacterial inoculant for plant growth stimulation under saline soil conditions. Acta Phys Plant 34:751–756
Egamberdieva D, Kucharova Z (2009) Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol Fertil Soils 45:561–573
Egamberdieva D, Berg G, Lindstrom K, Rasanen L (2010a) Root colonizing Pseudomonas spp. improve growth and symbiosis performance of fodder galega (Galega orientalis LAM) grown in potting soil. Eur J Soil Biol 46(3–4):269–272
Egamberdieva D, Renella G, Wirth S, Islam R (2010b) Secondary salinity effects on soil microbial biomass. Biol Fertil Soils 46(5):445–449
Egamberdieva D, Kucharova Z, Davranov K, Berg G, Makarova N, Azarova T, Chebotar V, Tikhonovich I, Kamilova F, Validov S, Lugtenberg B (2011) Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47:197–205
Egamberdieva D, Berg G, Lindström K, Räsänen LA (2013) Alleviation of salt stress of symbiotic Galega officinalis L. (goat's rue) by co-inoculation of Rhizobium with root colonizing Pseudomonas. Plant Soil 369(1):453–465
Egamberdieva D, Shurigin V, Gopalakrishnan S, Sharma R (2014) Growth and symbiotic performance of chickpea (Cicer arietinum) cultivars under saline soil conditions. J Biol Chem Res 31(1):333–341
Egamberdiyeva D, Gafurova L, Islam KR (2007) Salinity effects on irrigated soil chemical and biological properties in the syrdarya basin of Uzbekistan. In: Lal R, Sulaimanov M, Stewart B, Hansen D, Doraiswamy P (eds) Climate change and terrestrial c sequestration in Central Asia. Taylor-Francis, New York, pp 147–162
Essa TA (2002) Effect of salinity on growth and nutrient composition of three soybean (Glycine max L.) cultivars. J Agric Crop Sci 188:86–93
Estévez J, Dardanelli MS, Megias M, Rodríguez-Navarro DN (2009) Symbiotic performance of common bean and soybean co inoculated with rhizobia and Chryseobacterium balustinum Aur9 under moderate saline conditions. Symbiosis 49(1):29–36
FAOSTAT (2013) FAOSTAT database, Food and Agriculture Organization of the United Nations. http://faostat.fao.org/
Garg N, Chandel S (2011) Effect of mycorrhizal inoculation on growth, nitrogen fixation and nutrient uptake in Cicer arietinum L. under salt stress. Turk J Agric For 35:205–214
Golezani KG, Yengabad FM (2012) Physiological responses of lentil (Lens culinaris Medik.) to salinity. Int J Agric Crop Sci 4(20):1531–1535
Gunawardena SFBN, Danso SKA, Zapata F (1992) Phosphorus requirement and nitrogen accumulation by three mung bean (Vigna radiata (L.) Welzek) cultivars. Plant Soil 147:267–274
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hamayun M, Khan SA, Shinwari ZK, Khan AK, Ahmad N, Lee IJ (2010) Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pak J Bot 42:977–986
Hashem FM, Swelim DM, Kuykendall LD, Mohamed AI, Abdel-Wahab SM, Hegazi NI (1998) Identification and characterization of salt and thermo-tolerant Leucaena nodulating Rhizobium strains. Biol Fertil Soil 27:335–341
Hellsten A, Huss-Danell K (2001) Interaction effects of nitrogen and phosphorus on nodulation in red clover (Trifolium pretense L.). Acta Agric Scand Sect B Soil Plant Sci 50:135–142
Hiz MC, Canher B, Niron H, Turet M (2014) Transcriptome analysis of salt tolerant common bean (Phaseolus vulgaris L.) under saline conditions. PLoS One 9(3), e92598
Imai A, Matsuyama T, Hanzawa Y, Akiyama T, Tamaoki M, Saji H (2004) Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol 135:1565–1573
Imamul Huq SM, Larher F (1983) Osmoregulation in higher plants. Effect of NaCl salinity on non-nodulated Phaseolus aureus L. II. Changes in orgnaic solutes. New Phytol 93:209–216
Kaymakanova M (2009) Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol Equip 23:326–329
Khurana AS, Sharma P (2000) Effect of dual inoculation of phosphate solubilizing bacteria, Bradyrhizobium sp. and phosphorus on nitrogen fixation and yield of chickpea. Indian J Pulses Res 13:66–67
Lynch JP, Epstein E, Lauchli A, Weight GE (1990) An automated greenhouse sand culture system suitable for studies of P nutrition. Plant Cell Environ 13:547–554
Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar. viciae 1-amino cyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69:4396–4402
Molla AH, Shamsuddin ZH, Halimi MS, Morziah M, Puteh AB (2001) Potential for enhancement of root growth and nodulation of soybean coinoculated with Azospirillum and Bradyrhizobium in laboratory systems. Soil Biol Biochem 33:457–463
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chem Acta 27:31–36
Nabti E, Sahnoune M, Adjrad S, Van Dommelen A, Ghoul M, Schmid M, Hartmann A (2007) A halophilic and osmotolerant Azospirillum brasilense strain from Algerian soil restores wheat growth under saline conditions. Eng Life Sci 7(4):354–360
Naz I, Bano A (2012) Assessment of phytohormones producing capacity of Stenotrophomonas maltophilia SSA and its interaction with Zea mays l. Pak J Bot 44(1):465–469
Nelson DR, Mele PM (2007) Subtle changes in the rhizosphere microbial community structure in response to increased boron and sodium chloride concentrations. Soil Biol Biochem 39:340–351
Ofek M, Ruppel S, Waisel Y (2006) Effects of salinity on rhizosphere bacterial communities associated with different root types of Vicia faba L. In: Ozturk M, Waisel Y, Khan A, Gork G (eds) Biosaline agriculture and salinity tolerance in plants. Birkhauser Verlag, Basel, pp 1–21
Ondrasek G, Rengel Z, Romic D, Poljak M, Romic M (2009) Accumulation of non/essential elements in radish plants grown in salt-affected and cadmium contaminated environment. Cereal Res Commun 37:9–12
Park M, Kin C, Yang J, Lee Y, Shin W, Kim S, Sa T (2005) Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol Res 160:127–133
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants - a review. Plant Soil Environ 54(3):89
Qureshi MA, Shakir MA, Naveed M, Ahmad MJ (2009) Growth and yield response of chickpea to co-inoculation with Mesorhizobium ciceri and Bacillus megaterium. J Anim Plant Sci 19(4):205–211
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4(3):210–222
Roder A, Hoffmann E, Hagemann M, Berg G (2005) Synthesis of the compatible solutes glucosylglycerol and trehalose by salt-stressed cells of Stenotrophomonas strains. FEMS Microb Lett 243:219–226
Rokhzadi A, Asgharzadeh A, Darvish F, Nour-Muhammadi G, Majidi E (2008) Influence of plant growth promotingrhizobacteria on dry matter accumulation and yield of chickpea (Cicer arietinum L.) under field conditions. Am Eur J Agric Environ Sci 3(2):253–257
Rosas SB, Andres JA, Rovera M, Correa N (2006) Phosphate-solubilizing Pseudomonas putida can influence the rhizobia-legume symbiosis. Soil Biol Biochem 38:3502–3505
Schmidt CS, Alavi M, Cardinale M, Müller H, Berg G (2012) Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol Fertil Soils 48:947–960
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42(2):155–159
Shahzad SM, Khalid A, Arshad M, Rehman K (2010) Improving nodulation, growth and yield of Cicer arietinum L. through bacterial ACC-deaminase induced changes in root architecture. Eur J Soil Biol 46(5):342–347
Siddiqui ZA, Mahmood I (2001) Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Bioresour Technol 79(1):41–45
Simons M, van der Bij AJ, Brand I, de Weger LA, Wijffelman CA, Lugtenberg B (1996) Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol Plant-Microbe Interact 9:600–607
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic-acid in microbial and microorganism-plant signaling. FEMS Microb Rev 31:425–448
Subbarao GV, Johansen C, Jana MK, Rao JVDKK (1990) Physiological basis of differences in salinity tolerance of pigeonpea and its related wild species. J Plant Physiology 137(1):64–71
Suckstorff I, Berg G (2003) Evidence for dose-dependent effects on plant growth by Stenotrophomonas strains from different origins. J Appl Microbiol 95(4):656–663
Tisdall JM, Odes JM (1982) Organic matter and water stable aggregates in soils. J Soil Sci 33:141–163
UNEP (2008) In Dead Water. Merging of climate change with pollution, over-harvest, and infestations in the world’s fishing grounds. UNEP/GRID-Arendal, Arendal, Norway. Available online at: http://www.grida.no/_res/site/file/publications/InDeadWater_LR.pdf
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2:214–222
Vincent JM (1970) A manual for the practical study of root nodule bacteria LBP Handbook No.15. Blackwell, Oxford, p 83
Wolf A, Fritze A, Hagemann M, Berg G (2002) Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int J Syst Evol Microbiol 52:1937–1944
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Zhu B, Liu H, Tian WX, Fan XY, Li B, Zhou XP, Jin GL, Xie GL (2012) Genome Sequence of Stenotrophomonas maltophilia RR-10, Isolated as an endophyte from rice root. J Bacteriol 194(5):1280–1281
Acknowledgments
This study was supported by the UNESCO/CHINA Fellowship for DJ and Alexander von Humboldt Fellowship for DE. We thank Prof. Hong Liao for providing us necessary research facilities at Root Biology Centre, South China Agricultural University, and Muhammad Adam for technical assistance in the greenhouse.
Author contribution
DE and GB did experimental design work, DJ conducted experiments. DE analyzed the data. DE and GB wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Rights and permissions
About this article
Cite this article
Egamberdieva, D., Jabborova, D. & Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 405, 35–45 (2016). https://doi.org/10.1007/s11104-015-2661-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2661-8