Abstract
Key message
The OsMATE2 upon constitutive expression in tobacco decreases root-to-shoot As transfer coefficient and its endosperm-specific silencing in rice reduces grain As content, broadening the role of MATE proteins in planta.
Abstract
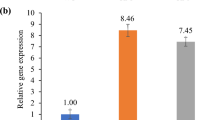
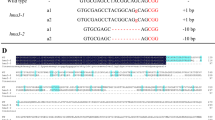
Rice (Oryza sativa) is capable of accumulating significant amount of arsenic (As) in grains, causing serious health hazard for rice consuming population. The multidrug and toxic compound extrusion (MATE) protein family comprises a large group of secondary transporters present universally in living organisms, and transports metabolites and/or xenobiotic compounds. OsMATE2, one of the MATE family members of rice was found to be transcriptionally up-regulated (sixfolds) in the developing seeds during As stress, and showed positive correlation with the As content in mature grains. Therefore, to understand the role of OsMATE2 in As accumulation, constitutive expression in tobacco was carried out. Transgenic tobacco plants exhibited decreased root-to-shoot As transfer coefficient (33.3–39.6%) along with augmented As sensitivity by increasing oxidative stress compared to untransformed control plants, indicating the involvement of OsMATE2 in As accumulation. Consequently, RNAi strategy was utilized for endosperm-specific silencing of endogenous OsMATE2 to mitigate As accumulation in rice grains. Transgenic rice lines demonstrated significant reduction of both OsMATE2 transcript (~ 38–87%) and grain As content (36.9–47.8%) compared to the control plants without undesirable effects on agronomical traits. Together, the present findings indicate the connection of OsMATE2 in As accumulation, and could expand the functional role of MATE proteins in planta.








Similar content being viewed by others

References
Banerjee J, Maiti MK (2010) Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochem Biophys Res Commun 394(1):178–183
Bashir K, Ishimaru Y, Shimo H, Kakei Y, Senoura T, Takahashi R, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011) Rice phenolics efflux transporter 2 (PEZ2) plays an important role in solubilizing apoplasmic iron. Soil Sci Plant Nutr 57(6):803–812
Bhattacharya S, Chattopadhyaya B, Koduru L, Das N, Maiti MK (2014) Bran-specific expression of Brassica juncea microsomal ω-3 desaturase gene (BjFad3) improves the nutritionally desirable ω-6:ω-3 fatty acid ratio in rice bran oil. Plant Cell Tiss Org 119(1):117–129
Bhattacharya S, Sinha S, Das N, Maiti MK (2015) Increasing the stearate content in seed oil of Brassica juncea by heterologous expression of MlFatB affects lipid content and germination frequency of transgenic seeds. Plant Physiol Biochem 96:345–355
Bhattacharya S, Das N, Maiti MK (2016) Cumulative effect of heterologous AtWri1 gene expression and endogenous BjAGPase gene silencing increases seed lipid content in Indian mustard Brassica juncea. Plant Physiol Biochem 107:204–213
Brown MH, Paulsen IT, Skurray RA (1999) The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol 31(1):394–395
Chen L, Liu Y, Liu H, Kang L, Geng J, Gai Y et al (2015) Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS ONE 10(3):e0118578
Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC et al (2000) Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108:393–397
Das N, Bhattacharya S, Maiti MK (2016) Enhanced cadmium accumulation and tolerance in transgenic tobacco overexpressing rice metal tolerance protein gene OsMTP1 is promising for phytoremediation. Plant Physiol Biochem 105:297–309
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2017) Identification of alternatively spliced transcripts of rice phytochelatin synthase 2 gene OsPCS2 involved in mitigation of cadmium and arsenic stresses. Plant Mol Biol 94(1–2):167–183
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F et al (2008) Phylogeny. fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(2):W465–W469
Dereeper A, Audic S, Claverie JM, Blanc G (2010) BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol 10(8):1
Diener AC, Gaxiola RA, Fink GR (2001) Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13(7):1625–1638
Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144(1):197–205
Finnegan P, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182
Fits VL, Deakin EA, Hoge JH, Memelink J (2000) The ternary transformation system: constitutive virG on a compatible plasmid dramatically increases Agrobacterium-mediated plant transformation. Plant Mol Biol 43:495–502
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K et al (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48(8):1081–1091
Green LS, Rogers EE (2004) FRD3 controls iron localization in Arabidopsis. Plant Physiol 136(1):2523–2531
Hartley-Whitaker J, Ainsworth G, Vooijs R, Ten Bookum W, Schat H, Meharg AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126(1):299–306
He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang Q et al (2010) Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 467(7318):991–994
Ishimaru Y, Bashir K, Nakanishi H, Nishizawa NK (2011a) The role of rice phenolics efflux transporter in solubilizing apoplasmic iron. Plant Signal Behav 6(10):1624–1626
Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011b) A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem 286(28):24649–24655
Kampfenkel K, Van Montagu M, Inzé D (1995) Effects of iron excess on Nicotiana plumbaginifolia plants (implications to oxidative stress). Plant Physiol 107:725–735
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Kuroda T, Tsuchiya T (2009) Multidrug efflux transporters in the MATE family. Biochim Biophys Acta Proteins Proteomics 1794(5):763–768
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57(3):389–399
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105(29):9931–9935
Magalhaes JV, Liu J, Guimaraes CT, Lana UG, Alves VM, Wang YH et al (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39(9):1156–1161
Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM et al (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19(6):2023–2038
Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N et al (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15(8):1689–1703
Meharg AA, Jardine L (2003) Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol 157(1):39–44
Miyauchi H, Moriyama S, Kusakizako T, Kumazaki K, Nakane T, Yamashita K, Hirata K, Dohmae N, Nishizawa T, Ito K, Miyaji T, Moriyama Y, Ishitani R, Nureki O (2017) Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat Commun 8:1633
Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T (1998) NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother 42:1778–1782
Morita Y, Kataoka A, Shiota S, Mizushima T, Tsuchiya T (2000) NorM of Vibrio parahaemolyticus is an Na(+)-driven multidrug efflux pump. J Bacteriol 182:6694–6697
Moriyama Y, Hiasa M, Matsumoto T, Omote H (2008) Multidrug and toxic compound extrusion (MATE)-type proteins as anchor transporters for the excretion of metabolic waste products and xenobiotics. Xenobiotica 38(7–8):1107–1118
Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14(1):275–286
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice–arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59(8):2267–2276
Otsuka M, Yasuda M, Morita Y, Otsuka C, Tsuchiya T, Omote H et al (2005) Identification of essential amino acid residues of the NorM Na+/multidrug antiporter in Vibrio parahaemolyticus. J Bacteriol 187:1552–1558
Rogers EE, Guerinot ML (2002) FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14(8):1787–1799
Sanyal SK (2005) Arsenic contamination in agriculture: a threat to water-soil-crop-animal-human continuum. Presidential address, section of agriculture & forestry sciences. In: 92th session of the Indian science congress association. Ahmedabad, Gujarat, pp 1–26
Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, Menzel DB (1999) A market basket survey of inorganic arsenic in food. Food Chem Toxicol 37(8):839–846
Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60:3419–3431
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Tiwari M, Sharma D, Singh M, Tripathi RD, Trivedi PK (2014) Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci Rep 4:3964
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12(3):364–372
Wang L, Bei X, Gao J, Li Y, Yan Y, Hu Y (2016) The similar and different evolutionary trends of MATE family occurred between rice and Arabidopsis thaliana. BMC Plant Biol 16:207
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41(19):6854–6859
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8(3):301–307
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149(1):297–305
Yokosho K, Yamaji N, Fujii-Kashino M, Ma JF (2016) Functional analysis of a MATE gene OsFRDL2 revealed its involvement in Al-induced secretion of citrate, but a lower contribution to Al tolerance in rice. Plant Cell Physiol 57(5):976–985
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181(4):777–794
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Acknowledgements
We sincerely thank late Prof. Soumitra K. Sen and Dr. Asitava Basu for their cooperation and help. The authors also acknowledge the help received from Dr. Tirthartha Chattopadhyay and Dr. Sheuli Roy during the initial phase of the study. We also acknowledge the technical help received from Mr. Sona Dogra, Mrs. Gayatri Aditya, Mr. Manoj Aditya and Mr. Nitai Giri. This work was supported by the grants from DBT, Govt. of India (BT/PR12907/AGR/36/639/2009), and the IIT Kharagpur Food Security Project (F. No. 4-25/2013-TS-1).
Author information
Authors and Affiliations
Contributions
Experimental designs and analyses of results were carried out by ND and MKM. ND conducted the experiments. SB1 helped ND in conducting few experiments and preparing the manuscript. MKM and SB2 conceived the original research plan. MKM made the necessary corrections in the manuscript and supervised the research work.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2018_766_MOESM1_ESM.tif
Supplementary figure S1. In silico analysis of the OsMATE2 polypeptide derived from the newly cloned OsMATE2 CDS of indica rice cultivar IR64. (A) Dompred server (http://bioinf.cs.ucl.ac.uk/psipred/?dompred=1) based prediction of the secondary structure of the OsMATE2 polypeptide. (B) MEMSAT-SVM server (http://bioinf.cs.ucl.ac.uk/psipred/?memsatsvm=1) mediated protein topology prediction using support vector machine-based (SVM) structures, highlighting the presence of 12 transmembrane helices in the OsMATE2 polypeptide. (TIF 1415 KB)
11103_2018_766_MOESM2_ESM.tif
Supplementary figure S2. Multiple sequence alignment of deduced amino acid sequences of OsMATE2 CDS with selected characterized MATE transporter orthologs, which are NorM of Vibrio cholerae, NorM of V. parahaemolyticus, ALF5 of A. thaliana, Jat1 of N. tabacum. The red boxes indicate the presence of the two amino acids E279 and D397 critical for cation binding in the OsMATE2 protein for the transporter activity. (TIF 3371 KB)
11103_2018_766_MOESM3_ESM.tif
Supplementary figure S3. Phylogenetic relationship of OsMATE2 using a few functionally characterized and non-characterized members of the MATE family proteins of plant kingdom including the representative members of both monocots and dicots. The unrooted phylogenetic tree was obtained on the basis of a Clustal Omega protein sequence alignment using the protein maximum likelihood (PhyML) algorithm. Analysis using 500 bootstrap replicates was performed. Scale bar indicates expected changes per site. (TIF 4782 KB)
11103_2018_766_MOESM4_ESM.tif
Supplementary figure S4. Homology modelling of OsMATE2 and in silico analysis. (A) The predicted model of OsMATE2 based on the crystal structure of AtDTX14 of A. thaliana (PDB ID 5Y50) showing the bi-lobed structure composed of N-lobe (TM 1-6) and C-lobe (TM 7-12) based on the arrangement of the 12 TM domains, forming a V-shaped structure open toward the extracellular side. (B) The position of the salt bridge in the predicted model of OSMATE2. (C) Interaction between Glu 103 (TM2) and Arg 408 (TM10) residues in the salt bridge, which may play critical role in transport activity of the protein by regulating the closing of the internal gate. (D) Position of the external gate in the predicted model of OsMATE2. (E) The external gate is composed of the residue pairs Asp 153 & Asn 295, and Ala 81 & Ser 304 along with the Gly 291, which together may contribute to the inward open structure of OsMATE2. (TIF 13548 KB)
11103_2018_766_MOESM5_ESM.tif
Supplementary figure S5. (A) EtBr stained agarose gel (0.8%) electrophoresis for verification of the pCAM::2xCaMV35S-OsMATE2-NOS recombinant binary plasmid by different restriction enzyme digestions. Lanes: 200 bp DNA ladder as molecular weight marker (lane 1), recombinant plasmid undigested (lane 2), and digested with BamHI+HindIII (lane 3), BamHI (lane 4), BamHI+KpnI (lane 5), KpnI (lane 6), SacI (lane 7) and XhoI (lane 8). (B) Representative Southern blot showing the integration pattern of the OsMATE2 transgene in T1 transgenic tobacco lines. Arrowheads indicate the position of the HindIII digested lambda DNA as a molecular weight marker. (C) EtBr stained agarose gel (0.8%) electrophoresis for verification of the pCAM::GluC-hpOsMATE2-NOS recombinant binary plasmid by different restriction enzyme digestions. Lanes: pUC18 DNA digested with HinfI as molecular weight marker (M), recombinant plasmid digested with HindIII (lane 1), BamHI (lane 2), BamHI+ HindIII (lane 3), PstI (lane 4) and KpnI (lane 5). (D) Representative Southern blot showing the integration pattern of the hp-OsMATE2 transgene in T2 transgenic rice lines. Lanes: untransformed control (Con), t independent T2 transgenic lines (hpL1- hpL6). Arrowheads indicate the position of HindIII digested lambda DNA as molecular weight marker. (TIF 10792 KB)
11103_2018_766_MOESM6_ESM.tiff
Supplementary figure S6. The universal expression pattern of rice MATE family genes and response of OsMATE2 in As stress of rice. (A) Metaexpression analysis of the MATE family genes in different anatomical tissues/organs or developmental stages of rice plant based on a large collection of Affymetrix microarray data (http://www.ricearray.org/). (B) Expression pattern of OsMATE2 (LOC_Os05g48040) in different anatomical tissues and in various developmental stages of rice plant. (C) Expression pattern of OsMATE2 (LOC_Os05g48040) in response to As treatment in the rice varieties Bala and Azucena, based on publicly available data (GSE4471, Norton et al. 2008). The numbers below the heat map indicate the log2 normalized intensity of the microarray data. (TIFF 2006 KB)
Rights and permissions
About this article
Cite this article
Das, N., Bhattacharya, S., Bhattacharyya, S. et al. Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol Biol 98, 101–120 (2018). https://doi.org/10.1007/s11103-018-0766-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0766-1