Abstract
Context
The recent WHO 2022 Classification of pituitary tumours identified a novel group of ‘plurihormonal tumours without distinct lineage differentiation (WDLD)’. By definition, these express multiple combinations of lineage commitment transcription factors, in a monomorphous population of cells.
Objectives
To determine the expression of stem cell markers (SOX2, Nestin, CD133) within tumours WDLD, immature PIT-1 lineage and acidophil stem cell tumours, compared with committed cell lineage tumours.
Methods
Retrospective evaluation of surgically resected pituitary tumours from St Vincent’s Hospital, Sydney. Patients were selected to cover a range of tumour types, based on transcription factor and hormone immunohistochemistry. Clinical data was collected from patient files. Radiology reports were reviewed for size and invasion. Samples were analysed by immunohistochemistry and RT-qPCR for SF-1, PIT-1, T-PIT, SOX2, Nestin and CD133. Stem cell markers were compared between tumours WDLD and those with classically “mature” types.
Results
On immunohistochemistry, SOX2 was positive in a higher proportion of tumours WDLD compared with those meeting WHO lineage criteria, 7/10 v 10/42 (70 v 23.4%, p = 0.005). CD133 was positive in 2/10 tumours WDLD but 0/41 meeting lineage criteria, P = 0.003. On RT-qPCR, there was no significant difference in relative expression of stem cell markers (SOX2, CD133, Nestin) between tumours with and WDLD.
Conclusions
Our study is the first to biologically characterise pituitary tumours WDLD. We demonstrate that these tumours exhibit a higher expression of the stem cell marker SOX2 compared with other lineage-differentiated tumours, suggesting possible involvement of stem cells in their development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During pituitary development, undifferentiated stem cells of the Rathke’s pouch generate all committed cell lineages of the anterior pituitary gland, following downregulation of stem cell transcription factors and upregulation of lineage specific transcription factors [1,2,3]. T-box transcription factor 19 (TBX19, T-PIT) expression occurs with development of corticotroph cells, steroidogenic factor 1 (NR5A1, SF-1) with development of gonadotroph cells and pituitary specific positive transcription factor 1 (POU1f1, PIT-1) with development to PIT-1 cell lineage, encompassing somatotroph, somatomammotroph, lactotroph and thyrotroph cells [1,2,3]. Committed progenitor cells are proliferative and give rise to hormone producing cells; which expand rapidly after birth. Ability of pituitary stem cells to proliferate and differentiate decreases with age into adulthood [1].
The role of stem cells in pituitary tumorigenesis has been the subject of debate. Pituitary tumours have classically been thought to be monoclonal in origin, based on evidence from X-chromosome inactivation studies and allelotype analyses [4, 5]. However, there is growing recognition of tumour heterogeneity and cell plasticity, observed in certain tumours, between primary and metastatic disease, and in hormonal transformation of tumour types [6, 7]. Advances in single cell technologies have facilitated a rapidly expanding body of evidence in this area, through spatially resolved cellular analyses and imaging [8].
Stem cells have been demonstrated to play a significant role in a number of malignancies such as leukaemia and solid organ cancers like breast cancer [9]. . The cancer stem cell theory proposes that neoplasia arise from tumour stem cells, maintain themselves by self-renewal and can “differentiate” to form different elements of the tumour mass [9]. The existence of cancer stem cells would account for observed intra-tumour heterogeneity; persistence and recurrence following apparent gross total resection and poor response to cytotoxic agents and radiotherapy, due to slow cell cycling and high expression of DNA repair proteins [9]. Several human pituitary tumour studies have isolated cells fulfilling some or all of the tumour stem cell criteria, specifically: ability to grow cell spheres in vitro; expression of stem cell markers and pituitary specific markers; ability to self-renew and to differentiate to form lineage specific hormone producing cells; cytotoxic resistance and in vitro tumourigenicity [9,10,11,12,13,14,15]. Within these studies, various pituitary stem cell markers have been studied. Three of the most consistently examined stem cell markers were SOX2, CD133 and Nestin [9,10,11,12,13,14,15]. SOX2 acts as a pluripotency inducing factor, which has an important role in pituitary stem cell multipotency [16]. Recent single nucleus transcriptomic data has demonstrated expression of SOX2 and SOX9 in uncommitted pituitary stem cells, whereas Nestin was expressed in a proportion of committed cells and was not universally expressed in pituitary stem cells [17].
The 2017 WHO Classification heralded a paradigm shift in the histopathological diagnosis of pituitary tumours, now based on cellular lineage, determined by transcription factor and hormonal immunohistochemistry [18]. Clinical application of transcription factor immunohistochemistry soon highlighted shortcomings within the new classification [19]. Notably, a previously undescribed group of tumours was identified, characterised by co-expression of two or more lineage-specific transcription factors. Co-expression of SF-1 and PIT-1 has also been reported in two case reports, wherein authors suggested that such tumours may represent poorly differentiated stem cell tumours [20, 21]. We have recently published on transcription factor analysis of 172 patients with pituitary tumours, in which we found 14 (8%) expressed both SF-1 and Pit-1 [19].
In the 2022 WHO Classification, a new category of pituitary tumours was named “Tumours without distinct lineage differentiation (WDLD)”, incorporating the newly defined “Plurihormonal tumours” along with previously defined “Null cell tumours”. The newly defined “Plurihormonal tumour” type was described as “very rare”, comprising a monomorphous population of cells that display features of multiple lineages [22]. The biology and prevalence of these tumours remains to be determined [20, 21, 23]. Although not classified as WDLD, another important change in the new classification was the distinction between mature plurihormonal PIT-1 lineage and immature PIT-1 lineage tumours [22].
Herein, we examined the expression of stem cell markers within WDLD, immature PIT-1 lineage and acidophil stem cell tumours, compared to classically ‘mature’ tumours with committed cell lineage. Additionally, we investigated the relationship between these tumours and markers of proliferation and invasion.
Materials and methods
Study design
This was a retrospective evaluation of surgically resected tumours from St Vincent’s Hospital, Sydney (2018_ETH00188). Ethical approval was obtained, complying with the Declaration of Helsinki. Patients were not required to provide consent, due to the retrospective and de-identified nature of our study.
Patient selection and data collection
Patients were selected (non-consecutively) to cover a range of different histopathological tumour types based on transcription factor immunohistochemistry (IHC): tumours expressing both SF-1 and PIT-1; tumours expressing SF-1 only; tumours expressing PIT-1 only; tumours expressing T-PIT only; PIT-1 lineage tumours (immature and mature plurihormonal) and normal pituitary tissue (control). Tumours expressing both SF-1 and PIT-1 were only selected if there was strong co-expression of each transcription factor. Patients were selected based on availability of tissue for immunohistochemistry and RT-qPCR analysis, favouring more recently operated tumours for sample quality. Clinical data was collected including patient demographics, presentation, tumour type and interventions (numbers of surgeries, radiotherapy, medical therapies). Radiology reports were reviewed for tumour size, cavernous sinus (CS) or sphenoid sinus (SS) invasion and remnant disease post-operatively. Histopathology reports were reviewed for results of haematoxylin and eosin (H&E) staining, hormonal immunohistochemistry (IHC), Ki67 and mitotic count.
Immunohistochemistry
Whole tumour Formalin-Fixed Paraffin-Embedded (FFPE) sections were cut and stained with antibodies for SF-1, PIT-1, T-PIT, SOX2, Nestin and CD-133. Antibody selection is summarised in Table 1, and was based on previous experience in pituitary tissue where available. Images were digitised with Roche DP200 slide scanner. Sections were reported for percentage of positive cells, staining intensity (strong, moderate, weak) and distribution with the assistance of tissue immunohistochemistry analysis software (Indica Labs, Halo) and three pituitary pathologists (JC, JL and PE). Stem cell markers were considered to be positive where ≥1% cells were positive.
Immunohistochemical evaluation of stem cell markers in tumours WDLD were compared with those meeting criteria for other WHO 2022 lineages. Subgroup analysis was performed to compare stem cell marker expression in WDLD tumours to those demonstrating clear lineage commitment: gonadotroph, lactotroph, somatotroph, mammosomatotroph, mixed somatotroph and lactotroph, thyrotroph, mature plurihormonal PIT-1 lineage and corticotroph, with the exclusion of tumours deemed “less mature” (Immature PIT-1 lineage and Acidophil stem cell tumours). There was one normal pituitary, obtained from a patient treated operatively for presumed pituitary tumour, with pathology yielding normal tissue only.
RT-qPCR
Tumour samples were preserved immediately (fresh frozen) after surgery and stored within the Garvan Institute Pituitary Tumour Biobank. RNA was extracted from tumour samples. cDNA synthesis was performed using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). cDNA was diluted in RNAse free water (Thermofisher). RT-qPCR was performed of transcription factor and stem cell markers as per previously described methods utilising primers listed in Table 1 [24]. Purchased pooled normal pituitary cDNA (Takarabio: Tissue-specific human cDNA; Human Pituitary Gland QUICK-Clone cDNA from 12 male/female Caucasians aged 18–52) was used with each run of RT-qPCR. RT-qPCR results were reported as tumour mRNA expression relative to pooled normal pituitary mRNA expression. RT-qPCR evaluation of stem cell markers in WDLD tumours were compared with those meeting criteria for other WHO 2022 lineages.
Statistical analysis
Results were presented as mean ± S.D. or median and interquartile range, as appropriate. Categorical variables were assessed by Chi-squared or Fisher’s exact test, while continuous variables which failed to satisfy parametric assumptions were assessed using the Mann-Whitney test. P < 0.05 was considered significant. Statistical analyses were carried out with SPSS version 27.
Results
There were 88 samples included in the study in total, of which 53/88 underwent immunohistochemical analysis, 72/88 underwent RT-qPCR analysis and 38/88 underwent analysis using both methods. There was 1 normal pituitary that was part of the immunohistochemistry group, a 24-year-old female, operated as a presumed non-functioning pituitary tumour. The normal pituitary was included for descriptive immunohistochemistry results but not subsequent analysis. Baseline characteristics are summarised in Table 2. Clinical diagnoses were as follows: 41 non-functioning (NFPT) (normal pituitary included in this number, given the presumed diagnosis prior to surgery), 21 acromegaly (GHoma), 13 prolactinoma (PRLoma), 10 Cushing’s (ACTHoma) and 3 TSH-secreting tumours (TSHoma). Median age was 52 years (Interquartile range (IQR) 37.3–64.0).
Immunohistochemistry group
There were 53 samples included in the analysis, including one normal pituitary. Baseline characteristics are summarised in Table 2. Clinical diagnoses were as follows: 21 NFPT, 16 GHoma, 8 PRLoma, 5 ACTHoma and 3 TSHoma. Complete immunohistochemistry was performed on normal pituitary. Stem cell markers, SOX2, CD133 and Nestin were negative in normal pituitary. It was then excluded from subsequent analysis. Median age was 50 (IQR 38.5–67.0) years. Cavernous and/or sphenoid sinus invasion was present in 18/53 (34%) patients. The WHO 2022 lineage type diagnoses were as follows: 14 gonadotroph (SF-1; all clinically NFPT); 6 lactotroph (Pit-1; all clinically PRLoma) ; 5 somatotroph (Pit-1; all clinically GHoma); 2 mammosomatotroph (Pit-1; 1 clinically GHoma and 1 clinically NFPT); 1 mixed somatotroph and lactotroph (Pit-1; clinically GHoma); 1 thyrotroph (Pit-1; clinically PRLoma); 1 mature PIT-1 lineage plurihormonal (Pit-1; clinically PRLoma); 6 immature PIT-1 lineage (Pit-1; 3 clinically GHoma and 3 clinically TSHoma); 1 acidophil stem cell (Pit-1; clinically NFPT); 5 corticotroph (T-Pit; all clinically ACTHoma); 1 null cell (clinically NFPT); 9 plurihormonal WDLD (7 clinically GHoma and 2 clinically NFPT). The 9 plurihormonal tumours WDLD were all strongly and diffusely positive for SF-1 and PIT-1. There was 1 tumour that did not meet criteria for WHO 2022 lineage type (clinically NFPT). This was a tumour that was negative for all transcription factors but expressed positive prolactin, TSH and growth hormone. There were 11 tumours with Ki67 ≥3% and 11 tumours with mitotic count ≥ 2/10 HPF; 15 tumours with Ki67 and/or mitotic count elevation and 7 tumours with both.
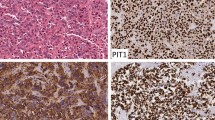
Immunohistochemical evaluation of stem cell markers in tumours with no WDLD (n = 10) were compared to those meeting criteria for other WHO 2022 lineages (n = 42) (Table 3). SOX2 was positive in a higher proportion of WDLD tumours compared with those meeting WHO lineage criteria, 7/10 (70%) v 10/42 (23.4%), P = 0.005 (Fig. 1). Of SOX2 positive WDLD tumours the percentage of SOX2 positive cells ranged from 1 to 46% with 6/7 exhibiting strongly positive staining intensity (Table 4). By comparison, the SOX2 positive tumours with distinct cell lineage demonstrated a lower range percentage of SOX2 positive (1–4%), with variable intensity of expression. CD133 was positive in 2/10 WDLD tumours but 0/41 tumours meeting lineage criteria, P = 0.003. Of these two CD133 positive tumours, one co-expressed SOX2 and the other did not. Percent positivity ranged from 1 to 2% of tumour cells. There was no significant difference between Nestin immunopositivity across groups (2/9 v 18/40, P 0.209).
Subgroup analysis was performed to compare stem cell marker expression in WDLD tumours to those demonstrating clear lineage commitment (n = 35) (Table 3). For this analysis, we excluded all Immature PIT-1 lineage (n = 6) and Acidophil stem cell tumours (n = 1). The aforementioned findings were upheld on this analysis, with SOX2 and CD133 both more expressed in WDLD tumours than those with committed lineage (p 0.006 and 0.056). We also compared both Immature PIT-1 lineage and Acidophil stem cell tumours with those with clear lineage commitment and found no difference in immunoexpression of stem cell markers (p 0.143 and 1.00 for SOX2).
Comparing WDLD with other tumours meeting lineage criteria we found no significant difference in tumour invasion between groups. There were no WDLD tumours with elevation in proliferative markers (Ki67 or mitotic count). In fact, 15/42 (36%) of tumours meeting lineage criteria had elevation in Ki67 or mitotic count (p = 0.025). Tumour invasiveness and proliferation were not associated with stem cell marker expression. Elevation of mitotic count was significantly associated with immunoexpression of Nestin, but not SOX2 or CD133.
RT-qPCR group
There were 72 samples that underwent RT-qPCR analysis. Baseline characteristics of the RT-qPCR group are summarised in Table 2. Clinical diagnoses were as follows: 34 NFPT, 19 GHoma, 6 ACTHoma, 10 PRLoma and 3 TSHoma. Median age was 52.5 (IQR 38.0–64.0). The WHO 2022 lineage type diagnoses were as follows: 23 gonadotroph, 7 lactotroph, 4 somatotroph, 1 mammosomatotroph, 3 mixed somatotroph and lactotroph, 1 thyrotroph, 1 mature plurihormonal, 7 immature PIT-1 lineage, 2 acidophil stem cell, 10 corticotroph, 13 plurihormonal tumours WDLD. There were 15 tumours (21.4%) with elevated Ki67 and 14 tumours (20%) with elevated mitotic count; overall 20 (27.8%) had elevation of Ki67 and/or mitotic count.
RT-qPCR evaluation of stem cell markers in WDLD tumours (n = 13) were compared with those meeting criteria for other WHO 2022 lineages (n = 59). There was no significant difference in relative mRNA expression of SOX2 (91.7 (24.9-172.9) v 59.2 (21.0-111.3), p = 0.244), Nestin (17.7 (6.3–34.6) v 14.8 (6.7–28.5), p = 0.884) or CD133 (4.2 (0.8–8.4) v 4.5 (1.3–26.1), p = 0.487) in the WDLD tumours compared with tumours meeting lineage criteria. Within the WDLD tumours, only 1/13 tumours had elevation of Ki67 and 0/13 tumours had elevation of mitotic count; the between group difference in proliferative marker expression did not reach statistical significance.
Comparison of transcription factor expression by IHC and RT-qPCR
Within the subgroup of tumours with complete IHC and RT-qPCR (n = 38), transcription factor protein expression on IHC was associated with relative mRNA expression on RT-qPCR (Table 5). In SF-1 lineage tumours (n = 12), relative SF-1 mRNA expression was elevated, but not significantly, whereas both PIT-1 and T-PIT expression were significantly suppressed. In PIT-1 lineage tumours (n = 16), relative PIT-1 mRNA expression was significantly elevated and T-PIT significantly suppressed. SF-1 expression was also present in Pit-1 lineage tumours but not statistically significantly higher than normal pituitary. In the single T-PIT lineage tumour, relative T-PIT mRNA expression was markedly elevated, PIT-1 was suppressed, and some SF-1 expression present. In the plurihormonal WDLD tumours (n = 9), there was no significant difference in relative mRNA expression of the transcription factors compared to normal pituitary.
Discussion
This is the first study to investigate and characterise tumours with “without distinct lineage differentiation”, as described in the 2022 WHO classification, by examination of stem cell marker expression and association with clinical parameters. Compared to pituitary tumours meeting clear lineage criteria, we found WDLD tumours more frequently expressed stem cell markers SOX2 and CD133 on IHC, the majority of which exhibited strong SOX2 intensity. Immature PIT-1 lineage tumours and Acidophil stem cell tumours did not demonstrate increased immunoexpression of stem cell markers. On RT-qPCR analysis, there was slightly higher relative mRNA expression of SOX2 and Nestin in tumours WDLD compared with others which did not reach statistical significance. Within both IHC and RT-qPCR groups, proliferative markers tended to be more frequently elevated in tumours meeting WHO 2022 lineage criteria, collectively but not individually reaching statistical significance. Larger numbers of tumours are required to determine if the non-significant results represent a type 2 statistical error.
Comparison between transcription factor expression by IHC and RT-qPCR demonstrated mixed results with regards to mRNA relative expression, however grossly reflected the immunohistochemistry findings without reaching statistical significance. Our results are consistent with previous findings that gonadotroph tumours expressed similar levels of SF-1 mRNA compared with other tumour types [24]. We hypothesise that this may reflect inclusion of normal cells within tissue collection for DNA extraction, skewing results. Additionally, there is evidence to suggest that discrepancy between gene and protein expression may relate to other factors such as miRNAs and mRNA stability. Based on our results and previous studies, we consider IHC to be the more reliable method for interpretation of transcription factors and stem cell markers [24].
In 2022, the WHO Classification defined a new type of tumour, “plurihormonal tumours WDLD”. By definition, these express multiple combinations of lineage commitment transcription factors, in a monomorphous population of cells [22]. Despite the inclusion in the WHO Classification, these tumours remain to be fully characterised and have henceforth been described only in case reports and one cohort study [20, 21, 23, 25]. The first reported a tumour co-expressing SF-1 and PIT-1 and proposed that multiple cellular commitment transcription factors within one population may arise in context of a collision tumour [21]. Subsequently, the same group reported another tumour co-expressing SF-1 and PIT-1, this time proposing that co-expression of multiple transcription factors may arise in a “poorly differentiated stem cell tumour” [20]. To our knowledge, this is the first study to characterise such tumours, and to evaluate stem cell markers on whole tumour sections.
Pituitary tumours are increasingly recognised as heterogenous neoplasms, with varying degrees of cellular maturity and plasticity. There is a growing body of literature proposing immaturity of “immature PIT-1 lineage” tumours, previously known as “silent subtype 3” and “PIT-1 plurihormonal” tumours [26,27,28,29,30]. Studies largely predate the adoption of transcription factor-based classification, necessitating further expansion of the literature, with more contemporary diagnostic techniques. Nonetheless, evidence suggests that these tumours are associated with progressive disease. Moreover, authors have postulated that these tumours might demonstrate a degree of “immaturity”, based on histopathological characteristics [26,27,28, 31,32,33]. However, in this study, we did not find higher expression of stem cell markers in these compared with other tumours, indicating that the term “immature” may not be an accurate reflection of tumour biology. To date, no other studies have investigated whether these tumours express stem cell markers.
There is a growing body of literature investigating cellular plasticity in pituitary tumours. In a recent study, SF-1 immunoexpression in gonadotroph tumour follicular cells was proposed to reflect neuroplasticity, specifically transformation of neoplastic cells into follicular cells [34]. Cancer stem cell theory postulates that tumours arise from cancer stem cells (CSC), which maintain by self-renewal and have multilineage potential, accounting for intratumour heterogeneity, persistence and recurrence [35]. CSC theory has been illustrated in other malignancies and more recently, there have been a number of human pituitary studies that have isolated cells fulfilling some or all of the CSC criteria [10,11,12,13,14,15]. However, recent mouse model studies suggest that stem cells do not directly generate tumourigenesis in the pituitary, rather they become activated in tumourigenesis, then fuel development and growth [35,36,37]. One study reported a stem cell compartment of SOX2 expressing cells, which was expanded in the early phase of the tumourigenic process [38]. Another study utilised immunofluorescence in human pituitary tumours to demonstrate expression of stem cell markers, SOX2 and SOX9, in a variety of clinical tumour types. SOX2 immunofluorescence was observed in tumours of different hormonal types (21/21) to varying degrees (0.05–38.24%), noting that transcription factors were not assessed. Stem cell markers, SOX2 and SOX9, were associated with increased proliferative status [3]. Interestingly, SOX2 immunofluorescence was detected in all (21/21) tumours, whereas SOX2 immunopositivity was detected in 17/55 tumours in the present study [3]. Differential expression between studies may be explained at least in part by the higher sensitivity of immunofluorescence compared with immunohistochemistry analysis. Another recent study compared gonadotroph tumours with varying levels of SF-1 IHC expression. SOX2 was upregulated on RNA sequencing in those tumours with low or patchy SF-1 expression when compared with those with diffuse SF-1 expression. Authors postulated that low SF-1 expression in gonadotroph tumours may represent intratumoural heterogeneity and lesser differentiation [39]. Further studies will help to refine IHC methods used to assess stem cells markers in human pituitary tumour tissue. Pituitary tumour stem cell marker expression has been associated with increased markers of proliferation, chemokines and cytokines, including IL-6, but not with clinically aggressive or metastatic disease, supporting a role in early tumourigenesis [3]. Our study identified increased stem cell marker but not proliferative marker expression in WDLD tumours, thereby supporting a possible role of stem cells in tumour formation and pituitary tumour cell differentiation. However, further studies are required to elucidate the role of stem cell marker positive cells within these tumours.
There are several strengths of this study. It is the first to characterise a series of WDLD Tumours and to investigate a possible role for stem cells in such tumours. We evaluated transcription factors and stem cell markers on whole sections, using digital imaging analysis technology and three pituitary pathologists with expertise in this field. We compared immunohistochemical findings with mRNA expression via RT-qPCR, though in our view, the latter appears to be a less robust method of assessment, both due to inclusion of bulk cells and variability between protein and mRNA expression. Future studies with spatially resolved profiling will help to elucidate stem cell marker expression and distribution. There are limitations of this study that must be acknowledged. Although we did not isolate tumour cells to confirm stem cell characteristics, previous human pituitary studies have demonstrated such findings in association with immunopositivity for markers CD133, SOX2 and Nestin. The selection of tumour samples was based on quality of cases, DNA and whole sections for the purposes of our analysis. This is associated with inherent selection bias and precludes prolonged follow-up. Future studies are required to investigate clinical outcomes in these tumours.
In conclusion, our study has characterised a novel type of pituitary tumour without distinct lineage differentiation. We have shown that WDLD tumours exhibit a higher expression of the stem cell marker SOX2, suggesting a possible involvement of stem cells in their development. Further studies are required to expand our characterisation of such tumours and to better understand the role of stem cells in early tumourigenesis of WDLD tumours. By expanding our knowledge in this field, we aim to develop scope for targeted therapies that may be used to treat tumours in early stages.
References
Russell JP, Lim X, Santambrogio A, Yianni V, Kemkem Y, Wang B, Fish M, Haston S, Grabek A, Hallang S, Lodge EJ, Patist AL, Schedl A, Mollard P, Nusse R, Andoniadou CL (2021) Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells. Elife 10. https://doi.org/10.7554/eLife.59142
Laporte E, Vennekens A, Vankelecom H (2020) Pituitary remodeling throughout life: are Resident Stem cells involved? Front Endocrinol (Lausanne) 11:604519. https://doi.org/10.3389/fendo.2020.604519
Nys C, Lee YL, Roose H, Mertens F, De Pauw E, Kobayashi H, Sciot R, Bex M, Versyck G, De Vleeschouwer S, Van Loon J, Laporte E, Vankelecom H (2022) Exploring stem cell biology in pituitary tumors and derived organoids. Endocr Relat Cancer 29(7):427–450. https://doi.org/10.1530/ERC-21-0374
Alexander JM, Biller BM, Bikkal H, Zervas NT, Arnold A, Klibanski A (1990) Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest 86(1):336–340. https://doi.org/10.1172/JCI114705
Herman V, Fagin J, Gonsky R, Kovacs K, Melmed S (1990) Clonal origin of pituitary adenomas. J Clin Endocrinol Metab 71(6):1427–1433. https://doi.org/10.1210/jcem-71-6-1427
Clayton RN, Farrell WE (2001) Clonality of pituitary tumours: more complicated than initially envisaged? Brain Pathol 11(3):313–327. https://doi.org/10.1111/j.1750-3639.2001.tb00402.x
Clayton RN, Pfeifer M, Atkinson AB, Belchetz P, Wass JA, Kyrodimou E, Vanderpump M, Simpson D, Bicknell J, Farrell WE (2000) Different patterns of allelic loss (loss of heterozygosity) in recurrent human pituitary tumors provide evidence for multiclonal origins. Clin Cancer Res 6(10):3973–3982
Cheung LY, Rizzoti K (2020) Cell population characterisation and discovery using single cell technologies in endocrine systems. J Mol Endocrinol. https://doi.org/10.1530/JME-19-0276
Haston S, Manshaei S, Martinez-Barbera JP (2018) Stem/progenitor cells in pituitary organ homeostasis and tumourigenesis. J Endocrinol 236(1):R1–R13. https://doi.org/10.1530/JOE-17-0258
Wurth R, Barbieri F, Pattarozzi A, Gaudenzi G, Gatto F, Fiaschi P, Ravetti JL, Zona G, Daga A, Persani L, Ferone D, Vitale G, Florio T (2017) Phenotypical and pharmacological characterization of Stem-Like cells in human pituitary adenomas. Mol Neurobiol 54(7):4879–4895. https://doi.org/10.1007/s12035-016-0025-x
Chen L, Ye H, Wang X, Tang X, Mao Y, Zhao Y, Wu Z, Mao XO, Xie L, Jin K, Yao Y (2014) Evidence of brain tumor stem progenitor-like cells with low proliferative capacity in human benign pituitary adenoma. Cancer Lett 349(1):61–66. https://doi.org/10.1016/j.canlet.2014.03.031
Xu Q, Yuan X, Tunici P, Liu G, Fan X, Xu M, Hu J, Hwang JY, Farkas DL, Black KL, Yu JS (2009) Isolation of tumour stem-like cells from benign tumours. Br J Cancer 101(2):303–311. https://doi.org/10.1038/sj.bjc.6605142
Mertens F, Gremeaux L, Chen J, Fu Q, Willems C, Roose H, Govaere O, Roskams T, Cristina C, Becú-Villalobos D, Jorissen M, Poorten VV, Bex M, Loon Jv, Vankelecom H (2015) Pituitary tumors contain a side population with tumor stem cell-associated characteristics. 22(4):481. https://doi.org/10.1530/erc-14-0546
Manoranjan B, Mahendram S, Almenawer SA, Venugopal C, McFarlane N, Hallett R, Vijayakumar T, Algird A, Murty NK, Sommer DD, Provias JP, Reddy K, Singh SK (2016) The identification of human pituitary adenoma-initiating cells. Acta Neuropathol Commun 4(1):125. https://doi.org/10.1186/s40478-016-0394-4
Peverelli E, Giardino E, Treppiedi D, Meregalli M, Belicchi M, Vaira V, Corbetta S, Verdelli C, Verrua E, Serban AL, Locatelli M, Carrabba G, Gaudenzi G, Malchiodi E, Cassinelli L, Lania AG, Ferrero S, Bosari S, Vitale G, Torrente Y, Spada A, Mantovani G (2017) Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int J Cancer 140(8):1870–1880. https://doi.org/10.1002/ijc.30613
Perez Millan MI, Cheung LYM, Mercogliano F, Camilletti MA, Chirino Felker GT, Moro LN, Miriuka S, Brinkmeier ML, Camper SA (2023) Pituitary stem cells: past, present and future perspectives. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-023-00922-4
Zhang Z, Zamojski M, Smith GR, Willis TL, Yianni V, Mendelev N, Pincas H, Seenarine N, Amper MAS, Vasoya M, Cheng WS, Zaslavsky E, Nair VD, Turgeon JL, Bernard DJ, Troyanskaya OG, Andoniadou CL, Sealfon SC, Ruf-Zamojski F (2022) Single nucleus transcriptome and chromatin accessibility of postmortem human pituitaries reveal diverse stem cell regulatory mechanisms. Cell Rep 38(10):110467. https://doi.org/10.1016/j.celrep.2022.110467
Lloyd RV, Kloppel OR, Rosai G (2017) J: WHO classification of tumours of endocrine organs, vol 10, 4 edn. IARC, Lyon
Lenders NF, Wilkinson AC, Wong SJ, Shein TT, Harvey RJ, Inder WJ, Earls PE, McCormack AI (2021) Transcription factor immunohistochemistry in the diagnosis of pituitary tumours. Eur J Endocrinol. https://doi.org/10.1530/EJE-20-1273
Tordjman KM, Greenman Y, Ram Z, Hershkovitz D, Aizenstein O, Ariel O, Asa SL (2019) Plurihormonal Pituitary Tumor of Pit-1 and SF-1 lineages, with Synchronous Collision Corticotroph Tumor: a possible stem cell phenomenon. Endocr Pathol 30(1):74–80. https://doi.org/10.1007/s12022-018-9562-3
Mete O, Alshaikh OM, Cintosun A, Ezzat S, Asa SL (2018) Synchronous multiple pituitary neuroendocrine tumors of different cell lineages. Endocr Pathol 29(4):332–338. https://doi.org/10.1007/s12022-018-9545-4
Asa SL, Mete O, Perry A, Osamura RY (2022) Overview of the 2022 WHO classification of Pituitary tumors. Endocr Pathol 33(1):6–26. https://doi.org/10.1007/s12022-022-09703-7
Tahara S, Kurotani R, Ishii Y, Sanno N, Teramoto A, Osamura RY (2002) A case of Cushing’s disease caused by pituitary adenoma producing adrenocorticotropic hormone and growth hormone concomitantly: aberrant expression of transcription factors NeuroD1 and Pit-1 as a proposed mechanism. Mod Pathol 15(10):1102–1105. https://doi.org/10.1097/01.MP.0000030451.28828.00
Torregrosa-Quesada ME, Garcia-Martinez A, Silva-Ortega S, Martinez-Lopez S, Camara R, Fajardo C, Lamas C, Aranda I, Pico A (2019) How Valuable is the RT-qPCR of Pituitary-Specific Transcription Factors for identifying Pituitary neuroendocrine tumor subtypes according to the New WHO 2017 Criteria? Cancers (Basel) 11(12). https://doi.org/10.3390/cancers11121990
Lenders NF, Chui J, Low J, Inder WJ, Earls PE, McCormack AI (2022) Development of a cost-effective diagnostic algorithm incorporating transcription factor immunohistochemistry in the evaluation of pituitary tumours. Pituitary 25(6):997–1003. https://doi.org/10.1007/s11102-022-01284-2
Horvath E, Kovacs K, Smyth HS, Cusimano M, Singer W (2005) Silent adenoma subtype 3 of the pituitary–immunohistochemical and ultrastructural classification: a review of 29 cases. Ultrastruct Pathol 29(6):511–524. https://doi.org/10.1080/01913120500323514
Erickson D, Scheithauer B, Atkinson J, Horvath E, Kovacs K, Lloyd RV, Young WF Jr. (2009) Silent subtype 3 pituitary adenoma: a clinicopathologic analysis of the Mayo Clinic experience. Clin Endocrinol (Oxf) 71(1):92–99. https://doi.org/10.1111/j.1365-2265.2008.03514.x
Mete O, Gomez-Hernandez K, Kucharczyk W, Ridout R, Zadeh G, Gentili F, Ezzat S, Asa SL (2016) Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod Pathol 29(2):131–142. https://doi.org/10.1038/modpathol.2015.151
Richardson TE, Shen ZJ, Kanchwala M, Xing C, Filatenkov A, Shang P, Barnett S, Abedin Z, Malter JS, Raisanen JM, Burns DK, White CL, Hatanpaa KJ (2017) Aggressive behavior in Silent Subtype III Pituitary Adenomas May depend on suppression of local Immune response: a whole transcriptome analysis. J Neuropathol Exp Neurol 76(10):874–882. https://doi.org/10.1093/jnen/nlx072
Fountas A, Lavrentaki A, Subramanian A, Toulis KA, Nirantharakumar K, Karavitaki N (2018) Recurrence in silent corticotroph adenomas after primary treatment: a systematic review and meta-analysis. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-01956
Richardson TE, Mathis DA, Mickey BE, Raisanen JM, Burns DK, White CL 3rd, Hatanpaa KJ (2015) Clinical outcome of Silent Subtype III Pituitary Adenomas diagnosed by immunohistochemistry. J Neuropathol Exp Neurol 74(12):1170–1177. https://doi.org/10.1097/NEN.0000000000000265
Pereira BD, Raimundo L, Mete O, Oliveira A, Portugal J, Asa SL (2016) Monomorphous Plurihormonal Pituitary Adenoma of Pit-1 lineage in a giant adolescent with central hyperthyroidism. Endocr Pathol 27(1):25–33. https://doi.org/10.1007/s12022-015-9395-2
Mete O, Lopes MB (2017) Overview of the 2017 WHO classification of Pituitary tumors. Endocr Pathol 28(3):228–243. https://doi.org/10.1007/s12022-017-9498-z
Delfin L, Mete O, Asa SL (2021) Follicular cells in pituitary neuroendocrine tumors. Hum Pathol 114:1–8. https://doi.org/10.1016/j.humpath.2021.05.002
Vankelecom H, Roose H (2017) The stem cell connection of Pituitary tumors. Front Endocrinol (Lausanne) 8:339. https://doi.org/10.3389/fendo.2017.00339
Gonzalez-Meljem JM, Haston S, Carreno G, Apps JR, Pozzi S, Stache C, Kaushal G, Virasami A, Panousopoulos L, Mousavy-Gharavy SN, Guerrero A, Rashid M, Jani N, Goding CR, Jacques TS, Adams DJ, Gil J, Andoniadou CL, Martinez-Barbera JP (2017) Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat Commun 8(1):1819. https://doi.org/10.1038/s41467-017-01992-5
Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP (2013) Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13(4):433–445. https://doi.org/10.1016/j.stem.2013.07.004
Mertens F, Gremeaux L, Chen J, Fu Q, Willems C, Roose H, Govaere O, Roskams T, Cristina C, Becu-Villalobos D, Jorissen M, Poorten VV, Bex M, van Loon J, Vankelecom H (2015) Pituitary tumors contain a side population with tumor stem cell-associated characteristics. Endocr Relat Cancer 22(4):481–504. https://doi.org/10.1530/ERC-14-0546
Hickman RA, Bruce JN, Otten M, Khandji AG, Flowers XE, Siegelin M, Lopes B, Faust PL, Freda PU (2021) Gonadotroph tumours with a low SF-1 labelling index are more likely to recur and are associated with enrichment of the PI3K-AKT pathway. Neuropathol Appl Neurobiol 47(3):415–427. https://doi.org/10.1111/nan.12675
Chang CV, Araujo RV, Cirqueira CS, Cani CM, Matushita H, Cescato VA, Fragoso MC, Bronstein MD, Zerbini MC, Mendonca BB, Carvalho LR (2017) Differential expression of stem cell markers in Human Adamantinomatous Craniopharyngioma and Pituitary Adenoma. Neuroendocrinology 104(2):183–193. https://doi.org/10.1159/000446072
Choi H, Kim Y, Kang D, Kwon A, Kim J, Kim M, Park J, Kim SS, Min YJ, Kim CK (2020) Common and different alterations of bone marrow mesenchymal stromal cells in myelodysplastic syndrome and multiple myeloma. Cell Prolif 53(5). https://doi.org/10.1111/cpr.12819. e12819
Virant-Klun I, Skerl P, Novakovic S, Vrtacnik-Bokal E, Smrkolj S (2019) Similar Population of CD133 + and DDX4 + VSEL-Like Stem cells sorted from human embryonic stem cell, ovarian, and ovarian Cancer ascites cell cultures. Real Embryonic Stem Cells? Cells 8(7). https://doi.org/10.3390/cells8070706
Acknowledgements
N F Lenders is supported by the University Post-graduate Award (University of New South Wales).
Funding
This research was supported by a SydPath Research Grant. NF Lenders is the recipient of grants from Ipsen and Pfizer and has received speaker honoraria with Novo Nordisk. The other authors have no relevant financial relationships to disclose.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
N.L. was responsible for study design, experimental work, data analysis, manuscript preparation and revision. P.E., A.M. and W.I. were involved in study design, data review and manuscript review. P.E., T.T., J.C. and J.L. were involved in experimental work and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lenders, N.F., Thompson, T.J., Chui, J. et al. Pituitary tumours without distinct lineage differentiation express stem cell marker SOX2. Pituitary (2024). https://doi.org/10.1007/s11102-024-01385-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11102-024-01385-0