Abstract
Aim
To determine the efficacy of diphenhydramine against cough due to respiratory infection or irritation in patients/subjects without comorbidities.
Method
Two reviewers independently identified English language studies, searching on: clinical trials, randomized, diphenhydramine (OR dimenhydrinate), antitussive agents, cough (combine using AND). Sources were: Medline (1966–2005), Embase (1980–2005), Cochrane and references from retrieved articles. Two other reviewers, blinded to study origin selected studies, inclusion criteria being: diphenhydramine monotherapy against placebo, double-blinded, randomized, clinical trial, intention-to-treat, dropout information. The blinded reviewers evaluated the selected studies on a quality scale.
Results
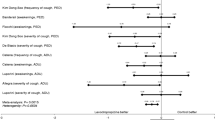
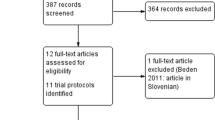
Eleven articles were identified, 7 were rejected (4 not placebo controlled, 2 had no diphenhydramine, 1 not blinded), leaving 4 articles, that were included in the evaluation and scored 20, 21, 25 and 26 out of a maximum of 32. In these selected studies, a total of 162 people were examined, 65 on diphenhydramine, 63 on placebo and 34 in a crossover setting. There was a total of 13 dropouts. The crossover studies demonstrated significant effect; 27–56% reduction in 20 healthy volunteers and 21–26% reduction in 13 patients (originally 14, one outlier left out), whereas the active versus placebo studies did not.
Conclusion
In spite of the 60 years that the substance has been on the market, only few studies have properly evaluated the effect of diphenhydramine against cough. Presumptions about efficacy of diphenhydramine against cough in humans are not univocally substantiated in literature.
Similar content being viewed by others
References
Anonymous. Parke Davis & Co. - Benylin Expectorant; Opportunity for Hearing on Proposal to Deny Approval of Supplemental New Drug Application. Docket No. 76–0483. Federal Register 1976;41(231):52537–9. U.S. FDA, Center for Drug Evaluation and Research. Available from: http://www.fda.gov/cder/otcmonographs/Cold&Cough/antitussive_benylin_expectorant_deny_approval_suppl_NDhttp://194.192.187.171/mdxcgi/prodindx.exe?CTL=/mdx/mdxcgi/MEGAT.SYS&SYS = 2&SET = 80A045834422809E38F00&POS = 7601795&T = 1614A_19761130.pdf Cited 19 May 2006.
Garnett WR. Diphenhydramine—A monograph. Am Pharm 1986;NS26&107(2):35–40.
MARTINDALE PRODUCT INDEX {diphenhydramine}. Available from Scandinavian Medical Information. http://www.smi.dk/index_mpas.html Cited 23 March 2006.
Sérlyfjaskrá (Medicinal Product Information). Icelandic Medicines Control Agency. Available from http://www.vefpostur.lyfjastofnun.is/focal/gnh52.nsf/TOC/24D67F0E6CD52B7F00256ECA003FF6A5/$FILE/Pektólín.doc Cited 19 May 2006.
Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA 1993;269:2258–63.
Lovell S. Cough medicines. On Continuing Practice 1982;9(4):21–4.
Katcher ML. Cold, cough, and allergy medications: uses and abuses. Pediatr Rev 1996;17(1):12–7.
Corrao WM. Chronic cough: an approach to management. Compr Ther 1986;12(7):14–9.
Phelan PD. Symptomatic treatment of cough in children. Curr Ther 1986;27/4:77–82.
Irwin RS, Curley FJ, Pratter MR. The effects of drugs on cough. Eur J Respir Dis 1987;71(Suppl.153):173–81.
Micromedex. Drug consults. Antitussive agents—comparative efficacy. Diphenhydramine. Available from Scandinavian Medical Information. http://www.smi.dk/index_mpas.html Cited 23 March 2006.
Banner AS. Cough: physiology, evaluation and treatment. Lung 1986;164:79–92.
Eddy NB, Friebel H, Hahn KJ, Halbach H. Codeine and its alternates for pain and cough relief. 3. The antitussive action of codeine–mechanism, methodology and evaluation. Bull World Health Organ 1969;40(3):425–454.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384.
Matts SG. A trial of Lotussin and linctus diphenhydramine in patients with an irritant cough. J Int Med Res 1977;5(6):470–2.
Packman EW, Ciccone PE, Wilson J, Masurat T. Antitussive effects of diphenhydramine on the citric acid aerosol-induced cough response in humans. Int J Clin Pharmacol Ther Toxicol 1991;29:218–22.
Lilienfield LS, Rose JC, Princiotto JV. Antitussive activity of diphenhydramine in chronic cough. Clin Pharmacol Ther 1976;19:421–5.
Danzon A, Lacroix J, Infante-Rivard C, Chicoine L. A double-blind clinical trial on Diphenhydramine in Pertussis. Acta Paediatr Scand 1988;77(4):614–5.
Paul IM, Yoder KE, et al. Effect of dextrometorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics 2004;114:85–90.
Weippl G. Therapeutic approaches to the common cold in children. Clin Ther 1984;6:475–82.
Middleton RSW. Double blind trial in general practice comparing the efficacy of “Benylin Day and Night” and paracetamol in the treatment of the common cold. Br J Clin Pract 1981;35:297–300.
Jaffe GV, Grimshaw JJ. Benylin Expectorant versus Actifed Expectorant in the treatment of acute cough. Br J Clin Pract 1985;39:238–42.
Jayaram S, Desai A. Efficacy and safety of Ascoril Expectorant and other cough formula in the treatment of cough management in paediatric and adult patients—a randomised double-blind comparative trial. J Indian Med Assoc 2000;98:68–70.
Curley FC, Irvin RS, Pratter MR, et al. Cough and the common cold. Am Rev Respir Dis 1988;138:305–11.
Hutton N, Wilson MH, Mellits D, et al. Effectiveness of an antihistamine-decongestant combination for young children with the common cold: a randomized, controlled clinical trial. J Pediatr 1991;118:125–30.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58(9):882–93.
Kleinschmidt H. [Attempt to test a new antitussive agent] [In German] Z Allgemeinmed. 1974;50(4):185–7.
Elberg M. [The treatment of dry cough in orhinolaryngology] [In German] ZFA (Stuttgart). 1976;52(10):542–3.
Weilacher JS [Cough and its treatment with a new cough-blocking drug] [In German]. Z Allgemeinmed. 1972;48(4):185–7.
Moher D, Jadad AR, Nichol G, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 1995;16:62–73.
Goodman SN, Berlin J, Fletcher SW, Fletcher RH Manuscript quality before and after peer review and editing at Annals of Internal Medicine. Ann Intern Med 1994;121(1):11–21.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding Necessary? Control Clin Trials 1996;17:1–12.
Directive 2004/24/Ec of The European Parliament and of The Council. Official Journal of the European Union 2004(30.4) L 136/85–90. Available from the homepage of European Commission, Enterprise and Industry, Pharmaceuticals at http://www.ec.europa.eu/enterprise/pharmaceuticals/review/doc/final_publ/dir_2004_24_20040430_en.pdf Cited 22 August 2006.
Acknowledgments
The study was done partly in the authors’ own time and partly while the first author was on a post doctoral fellow grant at the University of Toronto. The authors want to thank for constructive reviewer comments and editorial comments. The authors have no conflicts of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Study Inclusion Sheet to assess appropriateness of studies for meta-analysis/critical-review
Clinical trial | Yes | No | Do not know | ||
Blinding | Not | Single | Double | ||
English | Yes | No | Abstract English Not main text | ||
Subjects 1 year and older | Yes | No | Do not know | ||
Both genders | Yes | No | Do not know | ||
Diagnosis of cough, method: | |||||
Associated disorders | Yes | No | Do not know | ||
Patient entered on a previous date | Yes | No | Do not know | ||
Intention-to-treat study | Yes | No | Do not know | ||
Product with more than one active ingredient | Yes | No | Do not know | ||
Cough treatment with more than one product | Yes | No | Do not know | ||
Information about dropouts | Yes | No | Do not know | ||
Randomized | Yes | No | Do not know | ||
Comparison group | No treatment | Non-drug | Placebo | Other drug | Do not know |
Outcome | Success | Failure | Other | ||
Number of observations | |||||
Summaries of outcomes | |||||
Number of dropouts | |||||
Reasons for dropout | |||||
Appendix 2
Less-than-perfect quality: number of missing points* on the Downs & Black scale [14].
Rights and permissions
About this article
Cite this article
Björnsdóttir, I., Einarson, T.R., Guðmundsson, L.S. et al. Efficacy of diphenhydramine against cough in humans: a review. Pharm World Sci 29, 577–583 (2007). https://doi.org/10.1007/s11096-007-9122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-007-9122-2