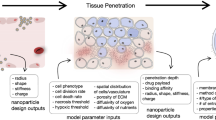
Abstract
Computational modeling of drug delivery is becoming an indispensable tool for advancing drug development pipeline, particularly in nanomedicine where a rational design strategy is ultimately sought. While numerous in silico models have been developed that can accurately describe nanoparticle interactions with the bioenvironment within prescribed length and time scales, predictive design of these drug carriers, dosages and treatment schemes will require advanced models that can simulate transport processes across multiple length and time scales from genomic to population levels. In order to address this problem, multiscale modeling efforts that integrate existing discrete and continuum modeling strategies have recently emerged. These multiscale approaches provide a promising direction for bottom-up in silico pipelines of drug design for delivery. However, there are remaining challenges in terms of model parametrization and validation in the presence of variability, introduced by multiple levels of heterogeneities in disease state. Parametrization based on physiologically relevant in vitro data from microphysiological systems as well as widespread adoption of uncertainty quantification and sensitivity analysis will help address these challenges.





Similar content being viewed by others
References
Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng. 2013;15(1):253–82.
Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D. Gold nanoparticles in cancer theranostics. Front Bioeng Biotechnol. 2021;13(9): 647905.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–17.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79.
Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16.
Ozcelikkale A, Ghosh S, Han B. Multifaceted transport characteristics of nanomedicine: needs for characterization in dynamic environment. Mol Pharm. 2013;10:2111–26.
Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98.
Sheth V, Wang L, Bhattacharya R, Mukherjee P, Wilhelm S. Strategies for delivering nanoparticles across tumor blood vessels. Adv Funct Mater. 2021;31(8):2007363.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
Ozcelikkale A, Moon H ran, Linnes M, Han B. In vitro microfluidic models of tumor microenvironment to screen transport of drugs and nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;e1460-n/a.
Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer. 2018;4(4):292–319.
Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A. 2013;110(46):18632–7.
Casalini T, Limongelli V, Limongelli V, Schmutz M, Som C, Jordan O, et al. Molecular modeling for nanomaterial-biology interactions: Opportunities, challenges, and perspectives. Vol. 7, Frontiers in Bioengineering and Biotechnology. Frontiers Media SA; 2019. p. 268–268.
Radhakrishnan R. Multiscale modeling: foundations, historical milestones, current status, and future prospects [Internet]. Preprints; 2020 Jun [cited 2021 Jul 28]. Available from: https://www.authorea.com/users/331603/articles/458216-multiscale-modeling-foundations-historical-milestones-current-status-and-future-prospects?commit=ea42454987ac2138e7acfc909f706b49037b9c7c
Ramezanpour M, Leung SSW, Delgado-Magnero KH, Bashe BYM, Thewalt J, Tieleman DP. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim Biophys Acta BBA - Biomembr. 2016;1858(7):1688–709.
Yong CW. Study of interactions between polymer nanoparticles and cell membranes at atomistic levels. Philos Trans R Soc B Biol Sci. 2015;370(1661):20140036.
Nguyen TD, Plimpton SJ. Aspherical particle models for molecular dynamics simulation. Comput Phys Commun. 2019;243:12–24.
Römer F, Kraska T. Molecular dynamics simulation of the formation of pharmaceutical particles by rapid expansion of a supercritical solution. J Supercrit Fluids. 2010;55(2):769–77.
Dror RO, Dirks RM, Grossman JP, Xu H, Shaw DE. Biomolecular simulation: a computational microscope for molecular biology. Annu Rev Biophys. 2012;41(1):429–52.
Curtarolo S, Ceder G. Dynamics of an inhomogeneously coarse grained multiscale system. Phys Rev Lett [Internet]. 2002; Available from: https://journals.aps.org/prl/abstract/https://doi.org/10.1103/PhysRevLett.88.255504
Ingólfsson H, Lopez C, Uusitalo J, ... The power of coarse graining in biomolecular simulations. Wiley … [Internet]. 2014; Available from: https://doi.org/10.1002/wcms.1169
Schiller UD, Krüger T, Henrich O. Mesoscopic modelling and simulation of soft matter. Soft Matter. 2018;14(1):9–26.
Abouali O, Nikbakht A, Ahmadi G, Saadabadi S. Three-dimensional simulation of brownian motion of nano-particles in aerodynamic lenses. Aerosol Sci Technol. 2009;43(3):205–15.
Liu Z, Zhu Y, Clausen JR, Lechman JB, Rao RR, Aidun CK. Multiscale method based on coupled lattice-Boltzmann and Langevin-dynamics for direct simulation of nanoscale particle/polymer suspensions in complex flows. Int J Numer Methods Fluids. 2019;91(5):228–46.
Erban R. From molecular dynamics to Brownian dynamics. Proc R Soc Math Phys Eng Sci. 2014;470(2167):20140036.
Ghosh PK, Hänggi P, Marchesoni F, Martens S, Nori F, Schimansky-Geier L, et al. Driven Brownian transport through arrays of symmetric obstacles. Phys Rev E. 2012;85(1): 011101.
Flegg MB, Rüdiger S, Erban R. Diffusive spatio-temporal noise in a first-passage time model for intracellular calcium release. J Chem Phys. 2013;138(15): 154103.
Vanden-Eijnden E, Venturoli M. Markovian milestoning with Voronoi tessellations. J Chem Phys. 2009;130(19): 194101.
Djohari H, Dormidontova EE. Kinetics of nanoparticle targeting by dissipative particle dynamics simulations. Biomacromol. 2009;10(11):3089–97.
Li Y, Kroeger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7(40):16631–46.
Kacar G. Molecular understanding of interactions, structure, and drug encapsulation efficiency of Pluronic micelles from dissipative particle dynamics simulations. Colloid Polym Sci. 2019;297(7–8):1037–51.
Alizadehrad D, Fedosov DA. Static and dynamic properties of smoothed dissipative particle dynamics. J Comput Phys. 2018;1(356):303–18.
Gompper G, Ihle T, Kroll DM, Winkler RG. Multi-particle collision dynamics -- a particle-based mesoscale simulation approach to the hydrodynamics of complex fluids. ArXiv08082157 Cond-Mat. 2009;1–87.
Padding J, Louis A. Hydrodynamic interactions and Brownian forces in colloidal suspensions: Coarse-graining over time and length scales. Phys Rev E [Internet]. 2006; Available from: https://doi.org/10.1103/PhysRevE.74.031402
Chen R, Poling-Skutvik R, P. Howard M, Nikoubashman A, A. Egorov S, C. Conrad J, et al. Influence of polymer flexibility on nanoparticle dynamics in semidilute solutions. Soft Matter. 2019;15(6):1260–8.
Nikoubashman A, N. Likos C, Kahl G. Computer simulations of colloidal particles under flow in microfluidic channels. Soft Matter. 2013;9(9):2603–13.
Bolintineanu DS, Grest GS, Lechman JB, Pierce F, Plimpton SJ, Schunk PR. Particle dynamics modeling methods for colloid suspensions. Comput Part Mech. 2014;1(3):321–56.
Batôt G, Dahirel V, Mériguet G, Louis AA, Jardat M. Dynamics of solutes with hydrodynamic interactions: comparison between Brownian dynamics and stochastic rotation dynamics simulations. Phys Rev E Stat Nonlin Soft Matter Phys. 2013;88(4): 043304.
Satō A. Introduction to practice of molecular simulation: molecular dynamics, Monte Carlo, Brownian dynamics, Lattice Boltzmann, dissipative particle dynamics. Amsterdam ; Boston: Elsevier; 2011. 322 p. (Elsevier insights).
Teeraratkul C, Mukherjee D. Microstructure aware modeling of biochemical transport in arterial blood clots. J Biomech. 2021;11(127): 110692.
Allaire G. Numerical analysis and optimization: an introduction to mathematical modelling and numerical simulation. Oxford ; New York: Oxford University Press; 2007. 455 p. (Numerical mathematics and scientific computation).
Peiró J, Sherwin S. Finite Difference, Finite Element and Finite Volume Methods for Partial Differential Equations. In: Yip S, editor. Handbook of Materials Modeling: Methods [Internet]. Dordrecht: Springer Netherlands; 2005 [cited 2021 Dec 15]. p. 2415–46. Available from: https://doi.org/10.1007/978-1-4020-3286-8_127
Jones DE, Ghandehari H, Facelli JC. A review of the applications of data mining and machine learning for the prediction of biomedical properties of nanoparticles. Comput Methods Programs Biomed. 2016;132:93–103.
Li M, Al-Jamal KT, Kostarelos K, Reineke J. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. Vol. 4, ACS Nano. American Chemical Society; 2010. p. 6303–17.
Jones HM, Rowland‐Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. Vol. 2, CPT: Pharmacometrics & Systems Pharmacology. Wiley-Blackwell; 2013. p. 1–12.
Utsey K, Gastonguay MS, Russell S, Freling R, Riggs MM, Elmokadem A. Quantification of the impact of partition coefficient prediction methods on physiologically based pharmacokinetic model output using a standardized tissue composition. Drug Metab Dispos. 2020;48(10):903–16.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Vol. 45, Clinical Pharmacokinectics. Springer International Publishing; 2006. p. 1013–34.
Buck SSD, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RAHJ. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Vol. 35, Drug Metabolism and Disposition. American Society for Pharmacology and Experimental Therapeutics; 2007. p. 1766–80.
Lankveld DPK, Oomen AG, Krystek P, Neigh A, Jong AT de, Noorlander CW, et al. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Vol. 31, Biomaterials. Elsevier; 2010. p. 8350–61.
Liu C, Xu XY. A systematic study of temperature sensitive liposomal delivery of doxorubicin using a mathematical model. Comput Biol Med. 2015;1(60):107–16.
Dubaj T, Kozics K, Sramkova M, Manova A, Bastús NG, Moriones OH, et al. Pharmacokinetics of PEGylated Gold Nanoparticles:In Vitro—In Vivo Correlation. 2022;12.
Zhang X, Yang Y, Grimstein M, Fan J, Grillo JA, Huang SM, et al. Application of PBPK modeling and simulation for regulatory decision making and its impact on us prescribing information: an update on the 2018–2019 submissions to the US FDA’s office of clinical pharmacology. J Clin Pharmacol. 2020;60(S1):S160–78.
Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc Natl Acad Sci [Internet]. 2018 Sep 11 [cited 2022 Mar 22];115(37). Available from: https://doi.org/10.1073/pnas.1807105115
Mould D, Upton R. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2(4):38.
McNally. A computational workflow for probabilistic quantitative in vitro to in vivo extrapolation. Front Pharmacol. 2018;
Liu Z, Zhu Y, Rao RR, Clausen JR, Aidun CK. Nanoparticle transport in cellular blood flow. Comput Fluids. 2018;172:609–20.
Lee TR, Greene MS, Jiang Z, Kopacz AM, Decuzzi P, Chen W, et al. Quantifying uncertainties in the microvascular transport of nanoparticles. Biomech Model Mechanobiol. 2014;13(3):515–26.
Zhang L, Gerstenberger A, Wang X, Liu WK. Immersed finite element method. Comput Methods Appl Mech Eng. 2004;193(21):2051–67.
Liu Y, Zhang L, Wang X, Liu WK. Coupling of navier-stokes equations with protein molecular dynamics and its application to hemodynamics. Int J Numer Methods Fluids. 2004;46(12):1237–52.
Li Y, Stroberg W, Lee TR, Kim HS, Man H, Ho D, et al. Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput Mech. 2014;53(3):511–37.
Park S, Whittington C, Voytik-Harbin SL, Han B. Microstructural parameter-based modeling for transport properties of collagen matrices. J Biomech Eng. 2015;137(6):0610031–9.
Schiller L, Naumann Z. A drag coefficient correlation. Ztg Ver Dtsch Ing. 1935;77:318–20.
Stylianopoulos T, Yeckel A, Derby JJ, Luo XJ, Shephard MS, Sander EA, et al. Permeability calculations in three-dimensional isotropic and oriented fiber networks. Phys Fluids. 2008;20(12): 123601.
Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci. 2016 Mar 1;113(9):E1142–51.
Islam MA, Barua S, Barua D. A multiscale modeling study of particle size effects on the tissue penetration efficacy of drug-delivery nanoparticles. BMC Syst Biol. 2017;11(1):113.
Barua D. A model-based analysis of tissue targeting efficacy of nanoparticles. J R Soc Interface. 2018;15(140):20170787.
Davit Y, Bell CG, Byrne HM, Chapman LAC, Kimpton LS, Lang GE, et al. Homogenization via formal multiscale asymptotics and volume averaging: How do the two techniques compare? Adv Water Resour. 2013;1(62):178–206.
Rim JE, Pinsky PM, Osdol WW van. Using the method of homogenization to calculate the effective diffusivity of the stratum corneum with permeable corneocytes. Vol. 41, Journal Of Biomechanics. Elsevier Sci Ltd; 2008. p. 788–96.
Muha I, Naegel A, Stichel S, Grillo A, Heisig M, Wittum G. Effective diffusivity in membranes with tetrakaidekahedral cells and implications for the permeability of human stratum corneum. Vol. 368, Journal Of Membrane Science. Elsevier Science Bv; 2011. p. 18–25.
Collis J, Hubbard ME, O’Dea RD. A multi-scale analysis of drug transport and response for a multi-phase tumour model. Vol. 28, European Journal Of Applied Mathematics. Cambridge Univ Press; 2017. p. 499–534.
Kremheller J, Vuong AT, Schrefler BA, Wall WA. An approach for vascular tumor growth based on a hybrid embedded/homogenized treatment of the vasculature within a multiphase porous medium model. Int J Numer Methods Biomed Eng. 2019;35(11): e3253.
Penta R, Ambrosi D. The role of the microvascular tortuosity in tumor transport phenomena. J Theor Biol. 2015;7(364):80–97.
Mascheroni P, Penta R. The role of the microvascular network structure on diffusion and consumption of anticancer drugs, vol. 33. International Journal For Numerical Methods In Biomedical Engineering: Wiley; 2017.
Kojic M, Milosevic M, Kojic N, Starosolski Z, Ghaghada K, Serda R, et al. A multi-scale FE model for convective–diffusive drug transport within tumor and large vascular networks. Comput Methods Appl Mech Eng. 2015;294:100–22.
Kojic M, Milosevic M, Simic V, Koay EJ, Fleming JB, Nizzero S, et al. A composite smeared finite element for mass transport in capillary systems and biological tissue. Comput Methods Appl Mech Eng. 2017;324:413–37.
Kojic M, Milosevic M, Kojic N, Koay EJ, Fleming JB, Ferrari M, et al. Mass release curves as the constitutive curves for modeling diffusive transport within biological tissue. Comput Biol Med. 2018;92:156–67.
Kojic M, Milosevic M, Simic V, Koay EJ, Kojic N, Ziemys A, et al. Multiscale smeared finite element model for mass transport in biological tissue: From blood vessels to cells and cellular organelles. Comput Biol Med. 2018;99:7–23.
Kannan R, Przekwas A. A multiscale absorption and transit model for oral drug delivery: Formulation and applications during fasting conditions. Int J Numer Methods Biomed Eng [Internet]. 2020 Mar [cited 2021 Oct 14];36(3). Available from: https://doi.org/10.1002/cnm.3317
He H, Liu C, Wu Y, Zhang X, Fan J, Cao Y. A multiscale physiologically-based pharmacokinetic model for doxorubicin to explore its mechanisms of cytotoxicity and cardiotoxicity in human physiological contexts, vol. 35. Pharmaceutical Research: Springer/Plenum Publishers; 2018.
Cordes H, Thiel C, Baier V, Blank LM, Kuepfer L. Integration of genome-scale metabolic networks into whole-body PBPK models shows phenotype-specific cases of drug-induced metabolic perturbation. Npj Syst Biol Appl. 2018;4(1):1–11.
Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59.
Veen LE, Hoekstra AG. Easing multiscale model design and coupling with MUSCLE 3. In: Krzhizhanovskaya VV, Závodszky G, Lees MH, Dongarra JJ, Sloot PMA, Brissos S, et al., editors. Computational Science – ICCS 2020. Cham: Springer International Publishing; 2020. p. 425–38. (Lecture Notes in Computer Science).
Eissing T, Kuepfer L, Becker C, Block M, Coboeken K, Gaub T, et al. A computational systems biology software platform for multiscale modeling and simulation: integrating whole-body physiology, disease biology, and molecular reaction networks. Front Physiol. 2011;2.
Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu Rev Chem Biomol Eng. 2011;2(1):281–98.
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer Nat Rev Dis Primer. 2016;2(1):16022.
Ottenhof NA, de Wilde RF, Maitra A, Hruban RH, Offerhaus GJ. Molecular characteristics of pancreatic ductal adenocarcinoma. Pathol Res Int. 2011/04/23 ed. 2011 Mar 27;2011:620601.
Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016/02/18 ed. 2016 Feb 15;30(4):355–85.
Karamitopoulou E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer. 2019;121(1):5–14.
Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, et al. Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance. Clin Cancer Res. 2018/04/18 ed. 2018 Sep 15;24(18):4444–54.
Choi SR, Yang Y, Huang KY, Kong HJ, Flick MJ, Han B. Engineering of biomaterials for tumor modeling. Mater Today Adv. 2020;1(8): 100117.
Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017/02/25 ed. 2017 Mar 6;214(3):579–96.
Tian C, Clauser KR, Ohlund D, Rickelt S, Huang Y, Gupta M, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U A. 2019/09/06 ed. 2019 Sep 24;116(39):19609–18.
Weniger M, Honselmann KC, Liss AS. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers Basel [Internet]. 2018/09/12 ed. 2018 Sep 6;10(9). Available from: https://www.ncbi.nlm.nih.gov/pubmed/30200666
Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015/10/20 ed. 2015 Dec;12(12):1197–204.
Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013/01/10 ed. 2013 Jan 15;108(1):1–8.
Sanh N, Fadul H, Hussein N, Lyn-Cook BD, Hammons G, Ramos-Cardona XE, et al. Proteomics Profiling of Pancreatic Cancer and Pancreatitis for Biomarkers Discovery. J Cell Sci Ther [Internet]. 2018/01/01 ed. 2018;9(4). Available from: https://www.ncbi.nlm.nih.gov/pubmed/31032145
Yang Y, Stang A, Schweickert PG, Lanman NA, Paul EN, Monia BP, et al. Thrombin Signaling Promotes Pancreatic Adenocarcinoma through PAR-1-Dependent Immune Evasion. Cancer Res. 2019/05/03 ed. 2019 Jul 1;79(13):3417–30.
Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015/02/25 ed. 2015 Apr;33(4):230–6.
Robinson BK, Cortes E, Rice AJ, Sarper M, Del Rio Hernandez A. Quantitative analysis of 3D extracellular matrix remodelling by pancreatic stellate cells. Biol Open. 2016/05/14 ed. 2016 Jun 15;5(6):875–82.
Rubiano A, Delitto D, Han S, Gerber M, Galitz C, Trevino J, et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 2017/12/02 ed. 2018 Feb;67:331–40.
Kihara T, Ito J, Miyake J. Measurement of biomolecular diffusion in extracellular matrix condensed by fibroblasts using fluorescence correlation spectroscopy. PLoS One. 2013/12/07 ed. 2013;8(11):e82382.
Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J. 2002/08/31 ed. 2002 Sep;83(3):1650–60.
Nieskoski MD, Marra K, Gunn JR, Hoopes PJ, Doyley MM, Hasan T, et al. Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Sci Rep. 2017/09/01 ed. 2017 Aug 30;7(1):10093.
Dedic J, Okur HI, Roke S. Hyaluronan orders water molecules in its nanoscale extended hydration shells. Sci Adv [Internet]. 2021/03/05 ed. 2021 Mar;7(10). Available from: https://www.ncbi.nlm.nih.gov/pubmed/33658208
Stromnes IM, DelGiorno KE, Greenberg PD, Hingorani SR. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014/06/09 ed. 2014 Jul;35(7):1451–60.
Andersen LMK, Wegner CS, Simonsen TG, Huang R, Gaustad JV, Hauge A, et al. Lymph node metastasis and the physicochemical micro-environment of pancreatic ductal adenocarcinoma xenografts. Oncotarget. 2017;8(29):48060–74.
Di Maggio F, Arumugam P, Delvecchio FR, Batista S, Lechertier T, Hodivala-Dilke K, et al. Pancreatic stellate cells regulate blood vessel density in the stroma of pancreatic ductal adenocarcinoma. Pancreatology. 2016;16(6):995–1004.
Jureidini R, da Cunha JEM, Takeda F, Namur GN, Ribeiro TC, Patzina R, et al. Evaluation of microvessel density and p53 expression in pancreatic adenocarcinoma. Clinics. 2016;71(6):315–9.
MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology. 1995;46(1):27–30.
Wang WQ, Liu L, Xu HX, Luo GP, Chen T, Wu CT, et al. Intratumoral α-SMA enhances the prognostic potency of CD34 associated with maintenance of microvessel integrity in hepatocellular carcinoma and pancreatic cancer. PLoS ONE. 2013;8(8): e71189.
Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147(1):9.
Weidner N. Measuring Intratumoral Microvessel Density. In: Methods in Enzymology [Internet]. Elsevier; 2008 [cited 2014 May 22]. p. 305–23. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0076687908028140
Gioeli D, Snow CJ, Simmers MB, Hoang SA, Figler RA, Allende JA, et al. Development of a multicellular pancreatic tumor microenvironment system using patient-derived tumor cells. Lab Chip. 2019/03/07 ed. 2019 Mar 27;19(7):1193–204.
Moon H ran, Han B. 15 - Engineered tumor models for cancer biology and treatment. In: Park K, editor. Biomaterials for Cancer Therapeutics (Second Edition) [Internet]. Woodhead Publishing; 2020. p. 423–43. Available from: http://www.sciencedirect.com/science/article/pii/B9780081029831000156
Nagy JA, Dvorak HF. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis. 2012/06/14 ed. 2012 Oct;29(7):657–62.
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2018/09/01 ed. 2019 Feb;22(1):15–36.
Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017/11/11 ed. 2017 Dec;17(12):738–50.
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014/05/27 ed. 2014 Jun 16;25(6):735–47.
Zhang X, Tian Y, Yang Y, Hao J. Development of anticancer agents targeting the Hedgehog signaling. Cell Mol Life Sci. 2017/03/21 ed. 2017 Aug;74(15):2773–82.
Doherty GJ, Tempero M, Corrie PG. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2017/12/14 ed. 2018 Jan;14(1):13–22.
Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun. 20181119th ed. 2018 Nov 19;9(1):4845.
Perus LJM, Walsh LA. Microenvironmental Heterogeneity in Brain Malignancies. Front Immunol. 2019;10:2294.
Boujelben A, Watson M, McDougall S, Yen YF, Gerstner ER, Catana C, et al. Multimodality imaging and mathematical modelling of drug delivery to glioblastomas. Interface Focus. 2016;6(5):20160039.
Terman D, Chen L, Hannawi Y. Mathematical modeling of cerebral capillary blood flow heterogeneity and its effect on brain tissue oxygen levels. J Theor Biol. 2021;527: 110817.
Bhandari A, Bansal A, Singh A, Sinha N. Numerical study of transport of anticancer drugs in heterogeneous vasculature of human brain tumors using dynamic contrast enhanced-magnetic resonance imaging. J Biomech Eng. 2018;140(5): 051010.
Bhandari A, Bansal A, Singh A, Gupta RK, Sinha N. Comparison of transport of chemotherapeutic drugs in voxelized heterogeneous model of human brain tumor. Microvasc Res. 2019;124:76–90.
Stapleton S, Mirmilshteyn D, Zheng J, Allen C, Jaffray DA. Spatial Measurements of Perfusion, Interstitial Fluid Pressure and Liposomes Accumulation in Solid Tumors. J Vis Exp [Internet]. 20160818th ed. 2016 Aug 18;(114). Available from: https://www.ncbi.nlm.nih.gov/pubmed/27583578
Howell B, McIntyre CC. Role of soft-tissue heterogeneity in computational models of deep brain stimulation. Brain Stimulat. 2017;10(1):46–50.
Larsson I. Modeling glioblastoma heterogeneity as a dynamic network of cell states. Mol Syst Biol. 2021;17(9):10105.
Carmona P, Mendez N, Ili CG, Brebi P. The Role of Clock Genes in Fibrinolysis Regulation: Circadian Disturbance and Its Effect on Fibrinolytic Activity. Front Physiol. 20200313th ed. 2020;11:129.
Hablitz LM, Pla V, Giannetto M, Vinitsky HS, Staeger FF, Metcalfe T, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 20200902nd ed. 2020 Sep 2;11(1):4411.
Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, et al. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun. 20210127th ed. 2021 Jan 27;12(1):617.
Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke J Cereb Circ. 1998;29(5):992–6.
Fodor DM, Marta MM, Perju-Dumbrava L. Implications of circadian rhythm in stroke occurrence: Certainties and possibilities. Brain Sci. 2021;11(7).
Verdi S, Marquand AF, Schott JM, Cole JH. Beyond the average patient: how neuroimaging models can address heterogeneity in dementia. Brain. 2021;144(10):2946–53.
Limbert G. Mathematical and computational modelling of skin biophysics: a review. Proc Math Phys Eng Sci. 2017;473(2203):20170257.
McLean K, Zhan W. Mathematical modelling of nanoparticle-mediated topical drug delivery to skin tissue. Int J Pharm. 2022;611: 121322.
Poorbahrami K, Mummy DG, Fain SB, Oakes JM. Patient-specific modeling of aerosol delivery in healthy and asthmatic adults. J Appl Physiol 1985. 20190912th ed. 2019 Dec 1;127(6):1720–32.
Sharma A, Merritt E, Hu X, Cruz A, Jiang C, Sarkodie H, et al. Non-genetic intra-tumor heterogeneity is a major predictor of phenotypic heterogeneity and ongoing evolutionary dynamics in lung tumors. Cell Rep. 2019 Nov 19;29(8):2164–2174 e5.
Tawhai M, Clark A, Donovan G, Burrowes K. Computational modeling of airway and pulmonary vascular structure and function: development of a “lung physiome.” Crit Rev Biomed Eng. 2011;39(4):319–36.
Whitfield CA, Horsley A, Jensen OE. Modelling structural determinants of ventilation heterogeneity: A perturbative approach. PLoS One. 20181129th ed. 2018;13(11):e0208049.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates. Infants and Children: Clin Pharmacokinet. 2006;45(9):931–56.
Cheng YH, He C, Riviere JE, Monteiro-Riviere NA, Lin Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. Vol. 14, ACS Nano. ACS Nano; 2020. p. 3075–95.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Vol. 51, Annual Review of Pharmacology and Toxicology. Annual Reviews; 2011. p. 45–73.
Yau E, Olivares-Morales A, Gertz M, Parrott N, Darwich AS, Aarons L, et al. Global sensitivity analysis of the rodgers and rowland model for prediction of tissue: plasma partitioning coefficients: assessment of the key physiological and physicochemical factors that determine small-molecule tissue distribution. AAPS J. 2020;22(2):41.
Pishko GL, Astary GW, Mareci TH, Sarntinoranont M. Sensitivity analysis of an image-based solid tumor computational model with heterogeneous vasculature and porosity. Ann Biomed Eng. 2011;39(9):2360–73.
Dalbey K, Eldred MS, Geraci G, Jakeman JD, Maupin KA, Monschke JA, et al. Dakota a multilevel parallel object-oriented framework for design optimization parameter estimation uncertainty quantification and sensitivity analysis: version 6.12 theory manual. [Internet]. Sandia National Lab. (SNL-NM), Albuquerque, NM (United States); 2020 May [cited 2021 Sep 30]. Report No.: SAND2020–4987. Available from: https://www.osti.gov/biblio/1630693-dakota-multilevel-parallel-object-oriented-framework-design-optimization-parameter-estimation-uncertainty-quantification-sensitivity-analysis-version-theory-manual
Marelli S, Sudret B. UQLab: A framework for uncertainty quantification in Matlab. 2014 Jul 7;2554–63.
Wang C, Duan Q, Tong CH, Di Z, Gong W. A GUI platform for uncertainty quantification of complex dynamical models. Environ Model Softw. 2016;1(76):1–12.
Patelli E. COSSAN: A multidisciplinary software suite for uncertainty quantification and risk management. In: Ghanem R, Higdon D, Owhadi H, editors. Handbook of Uncertainty Quantification [Internet]. Cham: Springer International Publishing; 2016 [cited 2021 Sep 30]. p. 1–69. Available from: https://doi.org/10.1007/978-3-319-11259-6_59-1
Hunt M, Haley B, McLennan M, Koslowski M, Murthy J, Strachan A. PUQ: A code for non-intrusive uncertainty propagation in computer simulations. Comput Phys Commun. 2015;1(194):97–107.
Verscheijden LFM, Koenderink JB, Johnson TN, Wildt SN de, Russel FGM. Physiologically-based pharmacokinetic models for children: Starting to reach maturation? Vol. 211, Pharmacology & Therapeutics. Pharmacol Ther; 2020. p. 107541.
Gampala S, Shah F, Lu X, Moon HR, Babb O, Umesh Ganesh N, et al. Ref-1 redox activity alters cancer cell metabolism in pancreatic cancer: exploiting this novel finding as a potential target. J Exp Clin Cancer Res CR. 2021;40(1):251.
Kwak B, Ozcelikkale A, Shin CS, Park K, Han B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J Controlled Release. 2014;28(194):157–67.
Moon H ran, Ozcelikkale A, Yang Y, Elzey BD, Konieczny SF, Han B. An engineered pancreatic cancer model with intra-tumoral heterogeneity of driver mutations. Lab Chip [Internet]. 2020 Sep 2 [cited 2020 Oct 6]; Available from: https://pubs.rsc.org/en/content/articlelanding/2020/lc/d0lc00707b
Ozcelikkale A, Shin K, Noe-Kim V, Elzey BD, Dong Z, Zhang JT, et al. Differential response to doxorubicin in breast cancer subtypes simulated by a microfluidic tumor model. J Controlled Release. 2017 Nov 28;266(Supplement C):129–39.
Shin K, Klosterhoff BS, Han B. Characterization of cell-type-specific drug transport and resistance of breast cancers using tumor-microenvironment-on-chip. Mol Pharm. 2016;13(7):2214–23.
Abaci HE, Shuler ML. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Vol. 7, Integrative Biology. The Royal Society of Chemistry; 2015. p. 383–91.
Ramadan Q, Fardous RS, Hazaymeh R, Alshmmari S, Zourob M. Pharmacokinetics-On-a-Chip: In Vitro Microphysiological Models for Emulating of Drugs ADME. Adv Biol. 2021;5(9):2100775.
Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng. 2020;4(4):421–36.
Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng. 2020;4(4):407–20.
Prantil-Baun R, Novak R, Das D, Somayaji MR, Przekwas A, Ingber DE. Physiologically based pharmacokinetic and pharmacodynamic analysis enabled by microfluidically linked organs-on-chips. Annu Rev Pharmacol Toxicol. 2018;58(1):37–64.
Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng. 2021;5(8):815–29.
Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The Design and Fabrication of Three‐Chamber Microscale Cell Culture Analog Devices with Integrated Dissolved Oxygen Sensors. Vol. 20, Biotechnology Progress. American Chemical Society (ACS); 2004. p. 338–45.
Vernetti L, Gough A, Baetz N, Blutt S, Broughman JR, Brown JA, et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep. 2017;7(1):42296.
Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol. 2013;5(9):1149–61.
Sung JH, Wang Y, Shuler ML. Strategies for using mathematical modeling approaches to design and interpret multi-organ microphysiological systems (MPS). APL Bioeng. 2019;3(2): 021501.
Adiwidjaja J, Boddy AV, McLachlan AJ. Implementation of a physiologically based pharmacokinetic modeling approach to guide optimal dosing regimens for imatinib and potential drug interactions in paediatrics. Front Pharmacol. 2020;30(10):1672.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet Syst Pharmacol. 2014;3(11):1–13.
Wikswo J, Curtis E, Eagleton Z, Evans B, Kole A, ... Scaling and systems biology for integrating multiple organs-on-a-chip [Internet]. Lab on a Chip. pubs.rsc.org; 2013. Available from: https://pubs.rsc.org/en/content/articlehtml/2013/lc/c3lc50243k
Maass C, Stokes CL, Griffith LG, Cirit M. Multi-functional scaling methodology for translational pharmacokinetic and pharmacodynamic applications using integrated microphysiological systems (MPS). Integr Biol. 2017;9(4):290–302.
Moradi Kashkooli F, Soltani M, Momeni MM. Computational modeling of drug delivery to solid tumors: A pilot study based on a real image. J Drug Deliv Sci Technol. 2021;62: 102347.
Zhan W. Convection enhanced delivery of anti-angiogenic and cytotoxic agents in combination therapy against brain tumour. Eur J Pharm Sci. 2020;141: 105094.
Lee CW, Stantz KM. Development of a mathematical model to estimate intra-tumor oxygen concentrations through multi-parametric imaging. Biomed Eng OnLine. 2016;15(1):114.
Bilgen M, Narayana PA. A pharmacokinetic model for quantitative evaluation of spinal cord injury with dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Med. 2001;46(6):1099–106.
Wang W, Ye Z, Gao H, Ouyang D. Computational pharmaceutics - A new paradigm of drug delivery. J Controlled Release. 2021;338:119–36.
Alber M, Buganza Tepole A, Cannon WR, De S, Dura-Bernal S, Garikipati K, et al. Integrating machine learning and multiscale modeling—perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. Npj Digit Med. 2019;2(1):115.
Peng GCY, Alber M, Buganza Tepole A, Cannon WR, De S, Dura-Bernal S, et al. Multiscale modeling meets machine learning: what can we learn? Arch Comput Methods Eng. 2021;28(3):1017–37.
Hataminia F, Noroozi Z, Mobaleghol EH. Investigation of iron oxide nanoparticle cytotoxicity in relation to kidney cells: A mathematical modeling of data mining. Toxicol In Vitro. 2019;59:197–203.
Findlay MR, Freitas DN, Mobed-Miremadi M, Wheeler KE. Machine learning provides predictive analysis into silver nanoparticle protein corona formation from physicochemical properties. Environ Sci Nano. 2018;5(1):64–71.
Sammut SJ, Crispin-Ortuzar M, Chin SF, Provenzano E, Bardwell HA, Ma W, et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature [Internet]. 2021 Dec 7 [cited 2021 Dec 13]; Available from: https://www.nature.com/articles/s41586-021-04278-5
Muñiz Castro B, Elbadawi M, Ong JJ, Pollard T, Song Z, Gaisford S, et al. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J Controlled Release. 2021;337:530–45.
Kojic M, Milosevic M, Kojic N, Kim K, Ferrari M, Ziemys A. A multiscale MD–FE model of diffusion in composite media with internal surface interaction based on numerical homogenization procedure. Comput Methods Appl Mech Eng. 2014;269:123–38.
Funding
This work was partially supported by grants from the National Institutes of Health (U01 HL143403, R01 CA254110, R61 HL159948 and P30 CA023168) and National Science Foundation (MCB-2134603) to BH and the Scientific and Technological Research Council of Turkey (TÜBİTAK 2232 118C200) to AO.
Author information
Authors and Affiliations
Contributions
AO and BH conceptualized the work. AAA, DDK and SRC performed the literature survey and drafted the manuscript. All authors made critical revisions and approved the version being submitted for review.
Corresponding authors
Ethics declarations
Conflicts of Interest Statement
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akalın, A.A., Dedekargınoğlu, B., Choi, S.R. et al. Predictive Design and Analysis of Drug Transport by Multiscale Computational Models Under Uncertainty. Pharm Res 40, 501–523 (2023). https://doi.org/10.1007/s11095-022-03298-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03298-8