Abstract
Purpose
Metal-organic frameworks (MOFs) have shown great potentials as drug delivery carriers, but the conventional methods for MOFs synthesis limited their use in biomedicine. The aim of this study was engineered tumor-targeted zinc nanoscale MOFs encapsulating chemotherapy drug.
Methods
We employed post-synthetic modification to construct tumor cell-targeted nanoscale zinc MOFs (nanoMOFs) functionalized with folate as the targeting ligand that binds specifically to folate receptors on tumor cells. The cytoctoxicity of drug-loaded nanoMOFs was measured by MTT assay. The cell target was tested by cell compete assay.
Results
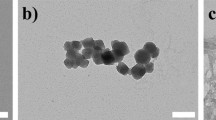
The successful synthesis of folate-targeted nanoMOFs was confirmed by FTIR, 1H-NMR and ESI-MS analysis. In the drug loading test, the zinc nanoMOFs functionalized with folate quickly adsorbed up to 24 wt % 5-fluorouracil (5-FU) without causing obvious changes in the supramolecular crystalline organization of the material. The in vitro drug release profile of this nanoMOFs in phosphate buffered saline exhibited a biphasic pattern. The drug sustained release is the effect mainly of diffusion of 5-FU molecules, while the degradation of the carrier itself plays a minor role in this process. The drug-loaded nanoMOFs showed a stronger cytotoxicity than free 5-FU against three cancer cell lines in vitro with a distinct selectivity between folate receptors positive and negative cells.
Conclusions
These results encourage further in vivo studies of this nanoMOFs as candidates for tumor-targeted, sustained-release delivery of chemotherapeutic agents.








Similar content being viewed by others
Abbreviations
- 5-FU:
-
5-fluorouracil
- 1 H NMR :
-
Proton nuclear magnetic resonance
- ATCC :
-
American Type Culture Collection
- CHCl 3 :
-
chloroform
- DCC :
-
N,N’-dicyclohexylcarbodiimide
- DMF :
-
Dimethyl formamide
- DMSO :
-
Dimethylsulf-oxide
- EA :
-
Elemental analysis
- ESI-MS :
-
Electrospray ionization-mass spectrometry
- FA-IRMOF-3 :
-
Folate-targeted zinc-based nanoMOFs
- FBS :
-
Fetal bovine serum
- FE-SEM :
-
Field emission scanning electron microscopy
- FR :
-
Folate receptors
- FT-IR :
-
Fourier transform infrared
- HPLC :
-
High-performance liquid chromatography
- IRMOF-3 :
-
Zn4O(C8H5NO4)3
- MOFs:
-
Metal-organic frameworks
- MTT :
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- nanoMOFs :
-
Nanosized MOFs
- NH 2 -BDC :
-
2-Aminobenzene-1,4-dicarboxylic acid
- PBS :
-
Phosphate buffered saline
- PXRD :
-
Powder X-ray diffraction
- SPSS :
-
Statistical Package for the Social Sciences
- TGA :
-
Thermogravimetric analysis
- UV :
-
Ultraviolet spectrophotometry
References
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, et al. Metal-organic frameworks in biomedicine. Chem Rev. 2012;112:1232–68.
Della Rocca J, Liu D, Lin W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44:957–68.
Cai W, Chu CC, Liu G, Wáng YX. Metal-organic framework-based nanomedicine platforms for drug delivery and molecular imaging. Small. 2015;11:4806–22.
Tan L, Li HW, Qiu YC, Chen DX, Wang X, Pan RY, et al. Stimuli-responsive metal–organic frameworks gated by pillar[5]arene supramolecular switches. Chem Sci. 2015;6:1640–4.
Tan L, Song N, Zhang SXA, Li HW, Wang B, Yang YW. Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J Mater Chem B. 2016;4:135–40.
Agostoni V, Chalati T, Horcajada P, Willaime H, Anand R, Semiramoth N, et al. Towards an improved anti-HIV activity of NRTI via metal-organic frameworks nanoparticles. Adv Healthc Mater. 2013;2:1630–7.
He CB, KD L, Liu DM, Lin WB. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136:5181–4.
di Nunzio MR, Agostoni V, Cohen B, Gref R, Douhal A. A “Ship in a bottle” strategy to load a hydrophilic anticancer drug in porous metal organic framework nanoparticles: efficient encapsulation, matrix stabilization, and photodelivery. J Med Chem. 2014;57:411-20.
Nagarkar SS, Desai AV, Ghosh SK. Stimulus-responsive metal-organic frameworks. Chem Asian J. 2014;9:2358–76.
Cohen SM. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem Rev. 2012;112:970–1000.
Garibay SJ, Wang ZQ, Tanabe KK, Cohen SM. Postsynthetic modification: a versatile approach toward multifunctional metal-organic frameworks. Inorg Chem. 2009;48:7341–9.
Bellido E, Hidalgo T, Lozano MV, Guillevic M, Simón-Vázquez R, Santander-Ortega MJ, et al. Heparin-engineered mesoporous iron metal-organic framework nanoparticles: toward stealth drug nanocarriers. Adv Healthc Mater. 2015;4:1246–57.
An J, Geib SJ, Rosi NL. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J Am Chem Soc. 2009;131:8376–7.
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172–8.
Ren F, Yang B, Cai J, Jiang YD, Xu J, Wang S. Toxic effect of zinc nanoscale metal-organic frameworks on rat pheochromocytoma (PC12) cells in vitro. J Hazard Mater. 2014;271:283–91.
Tan LL, Li H, Zhou Y, Zhang Y, Feng X, Wang B, et al. Zn2+-triggered drug release from biocompatible zirconium MOFs equipped with supramolecular gates. Small. 2015;11:3807–13.
YN W, Zhou M, Li S, Li Z, Li J, Wu B, et al. Magnetic metal-organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small. 2014;10:2927–36.
Ingleson MJ, Barrio JP, Guilbaud JB, Khimyak YZ, Rosseinsky MJ. Framework functionalisation triggers metal complex binding. Chem Commun. 2008;21:2680–2.
Wang ZQ, Tanabe KK, Cohen SM. Accessing postsynthetic modification in a series of metal-organic frameworks and the influence of framework topology on reactivity. Inorg Chem. 2009;48:296–306.
Werner ME, Copp JA, Karve S, Cummings ND, Sukumar R, Li C, et al. Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano. 2011;5:8990–8.
Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32:8548–54.
Sulistio A, Lowenthal J, Blencowe A, Bongiovanni MN, Ong L, Gras SL, et al. Folic acid conjugated amino acid-based star polymers for active targeting of cancer cells. Biomacromulecules. 2011;12:3469–77.
Pasut G, Canal F, Dalla VL, Arpicco S, Veronese FM, Schiavon O. Antitumoral activity of PEG-gemcitabine prodrugs targeted by folic acid. J Control Release. 2008;127:239–48.
Tanabe KK, Wang ZQ, Cohen SM. Systematic functionalization of a metal-organic framework via a postsynthetic modification approach. J Am Chem Soc. 2008;130:8508–17.
Zhang T, Li GY, Guo L, Chen H. Synthesis of thermo-sensitive CS-g-PNIPAM/CMC complex nanoparticles for controlled release of 5-FU. Int J Biol Macromol. 2012;51:1109–15.
Cheng MR, He B, Wan T, Zhu W, Han J, Zha B, et al. 5-fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS One. 2012;7:e47115.
Tse C, Zohdy MJ, Ye JY, O'Donnell M, Lesniak W, Balogh L. Enhanced optical breakdown in KB cells labeled with folate-targeted silver-dendrimer composite nanodevices. Nanomedicine. 2011;7:97–106.
Park EK, Lee SB, Lee YM. Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials. 2005;26:1053–61.
Au KM, Satterlee A, Min YZ, Tian X, Kim YS, Caster JM, et al. Folate-targeted pH-responsive calcium zoledronate nanoscale metalorganic frameworks: turning a bone antiresorptive agent into an anticancer therapeutic. Biomaterials. 2016;82:178-93.
Gao XC, Zhai MJ, Guan WH, Liu JJ, Liu ZL, Damirin A. Controllable synthesis of a smart multifunctional nanoscale metal–organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl Mater Interfaces. 2017;9:3455–62.
Hu Q, Yu J, Liu M, Liu A, Dou Z, Yang YA. Low cytotoxic cationic metal-organic framework carrier for controllable drug release. J Med Chem. 2014;57:5679–85.
Burns ER, Beland SS. Induction by 5-fluorouracil of a major phase difference in the circadian profiles of DNA synthesis between the Ehrlich ascites carcinoma and five normal organs. Cancer Lett. 1983;20:235–9.
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8.
Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther. 1990;48:381–95.
Francini G, Petrioli R, Aquino A, Gonnelli S. Advanced breast cancer treatment with folinic acid, 5-fluorouracil, and mitomycin C. Cancer Chemother Pharmacol. 1993;32:359–64.
Paolo A D, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol. 2001;12:1301–6.
van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Lenthe HV, De Abreu RA, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–12.
Lucena FR, de Araújo LC, Rodrigues DM, da Silva TG, Pereira VR, Militão GC, et al. Induction of cancer cell death by apoptosis and slow release of 5-fluoracil from metal-organic frameworks cu-BTC. Biomed Pharmacother. 2013;67:707–13.
Chowdhuri AR, Bhattacharya D, Sahu SK. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalton Trans. 2016;45:2963–73.
Lu Y, Ding N, Yang C, Huang L, Liu J, Xiang G. Preparation and in vitro evaluation of a folate-linked liposomal curcumin formulation. J Liposome Res. 2012;22:110–9.
Tsai PF, Chang WY, Hsiao YC, Li KJ, Shau MD. Synthesis and characterization of cationic glycidyl-based poly(aminoester)-folic acid targeting conjugates and study on gene delivery. Molecules. 2012;17:9056–69.
Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–8.
Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomark Prev. 1999;8:775–82.
Weitman SD, Frazier KM, Kamen BA. The folate receptor in central nervous system malignancies of childhood. J Neuro-Oncol. 1994;21:107–12.
Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VJ, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401.
Patrick TA, Kranz DM, van Dyke TA, Roy EJ. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J Neurooncol. 1997;32:111-23.
Zotter Á, Oláh J, Hlavanda E, Bodor A, Perczel A, Szigeti K, et al. Zn2+-induced rearrangement of the disordered TPPP/p25 affects its microtubule assembly and GTPase activity. Biochemistry. 2011;50:9568–78.
Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238.
Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998;15:587–627.
Acknowledgments and Disclosures
This work was funded by a Grant from the Science and Technology Planning Project of Guangdong Province (2014A010105026). This work was partially supported by grants from the National Natural Science Foundation of China (Grant 21,201,044), the Chinese Training Plan of Guangdong Province Outstanding Young Professors in Higher Education Institutions (GrantYQ2013084).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, B., Shen, M., Liu, J. et al. Post-Synthetic Modification Nanoscale Metal-Organic Frameworks for Targeted Drug Delivery in Cancer Cells. Pharm Res 34, 2440–2450 (2017). https://doi.org/10.1007/s11095-017-2253-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2253-9