Abstract
Purpose
To develop an in vitro methodology for prediction of concentrations and potential precipitation of highly permeable, lipophilic weak bases in fasted upper small intestine based on ketoconazole and dipyridamole luminal data. Evaluate usefulness of methodology in predicting luminal precipitation of AZD0865 and SB705498 based on plasma data.
Methods
A three-compartment in vitro setup was used. Depending on the dosage form administered in in vivo studies, a solution or a suspension was placed in the gastric compartment. A medium simulating the luminal environment (FaSSIF-V2plus) was initially placed in the duodenal compartment. Concentrated FaSSIF-V2plus was placed in the reservoir compartment.
Results
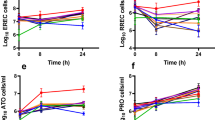
In vitro ketoconazole and dipyridamole concentrations and precipitated fractions adequately reflected luminal data. Unlike luminal precipitates, in vitro ketoconazole precipitates were crystalline. In vitro AZD0865 data confirmed previously published human pharmacokinetic data suggesting that absorption rates are not affected by luminal precipitation. In vitro SB705498 data predicted that significant luminal precipitation occurs after a 100 mg or 400 mg but not after a 10 mg dose, consistent with human pharmacokinetic data.
Conclusions
An in vitro methodology for predicting concentrations and potential precipitation in fasted upper small intestine, after administration of highly permeable, lipophilic weak bases in fasted upper small intestine was developed and evaluated for its predictability in regard to luminal precipitation.








Similar content being viewed by others
References
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the orange book database. J Med Chem. 2007;50(26):6665–72.
Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole: monitoring of intraluminal and plasma drug concentrations. Clin Pharmacokinet. 2011;50(11):725–34.
Box K, Comer JE, Gravestock T, Stuart M. New ideas about the solubility of drugs. Chem Biodivers. 2009;6(11):1767–88.
Bevernage J, Brouwers J, Clarysse S, Vertzoni M, Tack J, Annaert P, et al. Drug supersaturation in simulated and human intestinal fluids representing different nutritional states. J Pharm Sci. 2010;99(11):4525–34.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28(12):3145–58.
Sugano K. Computational oral absorption simulation of free base drugs. Int J Pharm. 2010;398(1–2):73–82.
Shono Y, Jantratid E, Dressman JB. Precipitation in the small intestine may play a more important role in the in vivo performance of poorly soluble weak bases in the fasted state: case example nelfinavir. Eur J Pharm Biopharm. 2011;79(2):349–56.
Carlert S, Pålsson A, Hanisch G, von Corswant C, Nilsson C, Lindfors L, et al. Predicting intestinal precipitation-a case example for a basic BCS class II drug. Pharm Res. 2010;27(10):2119–30.
Kobayashi M, Sada N, Sugawara M, Iseki K, Miyazaki K. Development of a new system for prediction of drug absorption that takes into account drug dissolution and pH change in the gastro-intestinal tract. Int J Pharm. 2001;221(1–2):87–94.
Kostewicz ES, Wunderlich M, Brauns U, Becker R, Bock T, Dressman JB. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J Pharm Pharmacol. 2004;56(1):43–51.
Gu CH, Rao D, Gandhi RB, Hilden J, Raghavan K. Using a novel multicompartment dissolution system to predict the effect of gastric pH on the oral absorption of weak bases with poor intrinsic solubility. J Pharm Sci. 2005;94(1):199–208.
Bhattachar SN, Perkins EJ, Tan JS, Burns LJ. Effect of gastric pH on the pharmacokinetics of a BCS class II compound in dogs: utilization of an artificial stomach and duodenum dissolution model and Gastroplus™ simulations to predict absorption. J Pharm Sci. 2011;100(11):4756–65.
Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132(1–2):132–41.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
Kelly K, O’Mahony B, Lindsay B, Jones T, Grattan TJ, Rostami-Hodjegan A, et al. Comparison of the rates of disintegration, gastric emptying, and drug absorption following administration of a new and a conventional paracetamol formulation, using gamma scintigraphy. Pharm Res. 2003;20(10):1668–73.
Oberle RL, Chen TS, Lloyd C, Barnett JL, Owyang C, Meyer J, et al. The influence of the interdigestive migrating myoelectric complex on the gastric emptying of liquids. Gastroenterology. 1990;99(5):1275–82.
Kalantzi L, Persson E, Polentarutti B, Abrahamsson B, Goumas K, Dressman JB, et al. Canine intestinal contents vs. simulated media for the assessment of solubility of two weak bases in the human small intestinal contents. Pharm Res. 2006;23(6):1373–81.
Vertzoni M, Pastelli E, Psachoulias D, Kalantzi L, Reppas C. Estimation of intragastric solubility of drugs: in what medium? Pharm Res. 2007;24(5):909–17.
Schiller C, Fröhlich CP, Giessmann T, Siegmund W, Mönnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2005;22(10):971–9.
Huang YC, Colaizzi JL, Bierman RH, Woestenborghs R, Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob Agents Chemother. 1986;30(2):206–10.
Endrenyi L, Fritsch S, Yan W. Cmax/AUC is a clearer measure than Cmax for absorption rates in investigations of bioequivalence. Int J Clin Pharmacol Ther Toxicol. 1991;29(10):394–9.
Lacey LF, Keene ON, Duquesnoy C, Bye A. Evaluation of different indirect measures of rate of drug absorption in comparative pharmacokinetic studies. J Pharm Sci. 1994;83(2):212–5.
Chen M-L. An alternative approach for assessment of rate of absorption in bioequivalence studies. Pharm Res. 1992;9(11):1380–5.
Macheras P, Symillides M, Reppas C. The cutoff time point of the partial area method for assessment of rate of absorption in bioequivalence studies. Pharm Res. 1994;11(6):831–4.
Acknowledgments And Disclosures
Authors would like to thank B. Abrahamsson (Pharmaceutical Development, AstraZeneca R&D, Mölndal, Sweden) for providing the AZD0865 material and valuable comments, N. Koumandrakis and M. Dimopoulou (Department of Pharmaceutical Technology, National and Kapodistrian University of Athens, Athens, Greece) for their assistance in the evaluation of FaSSIF-V2plus, and S. Beato (Phad oral dosage forms 1, Novartis Pharma AG, Basel, Switzerland) for valuable comments during the design of the in vitro experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Psachoulias, D., Vertzoni, M., Butler, J. et al. An In Vitro Methodology for Forecasting Luminal Concentrations and Precipitation of Highly Permeable Lipophilic Weak Bases in the Fasted Upper Small Intestine. Pharm Res 29, 3486–3498 (2012). https://doi.org/10.1007/s11095-012-0844-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0844-z