Abstract
Purpose
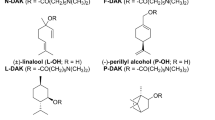
Series of N,N-dimethylamino acid esters was synthesized to study their transdermal permeation-enhancing potency, biodegradability and reversibility of action. Effects of chirality, linking chain length and polyfluorination were investigated.
Materials and Methods
In vitro activities were evaluated using porcine skin and four model drugs—theophylline, hydrocortisone, adefovir and indomethacin. Biodegradability was determined using porcine esterase, reversibility was measured using electrical resistance.
Results
No differences in activity were found between (R), (S) and racemic dodecyl 2-(dimethylamino)propanoate (DDAIP). Substitution of hydrocarbon tail by fluorocarbon one resulted in loss of activity. Replacement of branched linking chain between nitrogen and ester of DDAIP by linear one markedly improved penetration-enhancing activity with optimum in 4–6C acid derivatives. Dodecyl 6-(dimethylamino)hexanoate (DDAK) was more potent than clinically used skin absorption enhancer DDAIP for theophylline (enhancement ratio of DDAK and DDAIP was 17.3 and 5.9, respectively), hydrocortisone (43.2 and 11.5) and adefovir (13.6 and 2.8), while DDAIP was better enhancer for indomethacin (8.7 and 22.8). DDAK was rapidly metabolized by porcine esterase, and displayed low acute toxicity. Electrical resistance of DDAK-treated skin barrier promptly recovered to control values.
Conclusion
DDAK, highly effective, broad-spectrum, biodegradable and reversible transdermal permeation enhancer, is promising candidate for future research.








Similar content being viewed by others
References
M. R. Prausnitz, S. Mitragotri, and R. Langer. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 3:115–124 (2004). doi:10.1038/nrd1304.
B. J. Thomas, and B. C. Finnin. The transdermal revolution. Drug Discov. Today. 9:697–703 (2004). doi:10.1016/S1359-6446(04)03180-0.
H. Y. Thong, H. Zhai, and H. I. Maibach. Percutaneous penetration enhancers: an overview. Skin Pharmacol. Physiol. 20:272–282 (2007). doi:10.1159/000107575.
A. C. Williams, and B. W. Barry. Penetration enhancers. Adv. Drug Deliv. Rev. 56:603–618 (2004). doi:10.1016/j.addr.2003.10.025.
K. Vavrova, J. Zbytovska, and A. Hrabalek. Amphiphilic transdermal permeation enhancers: structure-activity relationships. Curr. Med. Chem. 12:2273–2291 (2005). doi:10.2174/0929867054864822.
S. Buyuktimkin, N. Buyuktimkin, and J. H. Rytting. Synthesis and enhancing effect of dodecyl 2-(N,N-dimethylamino)propionate on the transepidermal delivery of indomethacin, clonidine, and hydrocortisone. Pharm. Res. 10:1632–1637 (1993). doi:10.1023/A:1018980905312.
T. M. Suhonen, L. Pirskanen, M. Raisanen, K. Kosonen, J. H. Rytting, P. Paronen, and A. Urtti. Transepidermal delivery of beta-blocking agents: Evaluation of enhancer effects using stratum corneum lipid liposomes. J. Control. Release. 43:251–259 (1997). doi:10.1016/S0168-3659(96)01495-2.
A. M. Wolka, J. H. Rytting, B. L. Reed, and B. C. Finnin. The interaction of the penetration enhancer DDAIP with a phospholipid model membrane. Int. J. Pharm. 271:5–10 (2004). doi:10.1016/j.ijpharm.2003.09.018.
T. M. Turunen, A. Urtti, P. Paronen, K. L. Audus, and J. H. Rytting. Effect of some penetration enhancers on epithelial membrane lipid domains: evidence from fluorescence spectroscopy studies. Pharm. Res. 11:288–294 (1994). doi:10.1023/A:1018919811227.
N. Buyuktimkin, S. Buyuktimkin, and J. H. Rytting. Alkyl N,N-Disubstituted-Amino acetates. In E. W. Smith, and H. I. Maibach (eds.), Percutaneous Penetration Enhancers, CRC, New York, 1995, pp. 91–102.
S. Buyuktimkin, N. Buyuktimkin, and J. H. Rytting. Interaction of indomethacin with a new penetration enhancer, dodecyl 2-(N,N-dimethylamino)propionate (DDAIP): Its effect on transdermal delivery. Int. J. Pharm. 127:245–253 (1996). doi:10.1016/0378–5173(96)80691-0.
W. Pfister, M. Li, and D. Frank. Development of the novel permeation enhancers dodecyl-2-N,N-dimethylaminopropionate (DDAIP) and HCl salt: physiochemical properties, preclinical safety and in vitro permeation enhancement. AAPS J. 8:(2006).
E. Touitou, B. Godin, T. R. Kommuru, M. I. Afouna, and I. K. Reddy. Transport of chiral molecules across the skin. In I. K. Reddy, and R. Mehvar (eds.), Chirality in Drug Design and Development, Marcel Dekker, New York, 2004, pp. 67–99.
K. Vavrova, A. Hrabalek, and P. Dolezal. Enhancement effects of (R) and (S) enantiomers and the racemate of a model enhancer on permeation of theophylline through human skin. Arch. Dermatol. Res. 294:383–385 (2002).
N. Kanikkannan, K. Kandimalla, S. S. Lamba, and M. Singh. Structure–activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr. Med. Chem. 7:593–608 (2000).
K. Vavrova, A. Hrabalek, P. Dolezal, T. Holas, and J. Klimentova. Biodegradable derivatives of tranexamic acid as transdermal permeation enhancers. J. Control. Release. 104:41–49 (2005). doi:10.1016/j.jconrel.2005.01.002.
P. Vierling, C. Santaella, and J. Greiner. Highly fluorinated amphiphiles as drug and gene carrier and delivery systems. J. Fluorine Chem. 107:337–354 (2001). doi:10.1016/S0022-1139(00)00378-X.
K. Wang, G. Karlsson, M. Almgren, and T. Asakawa. Aggregation behavior of cationic fluorosurfactants in water and salt solutions. A cryoTEM survey. J. Phys. Chem. B. 103:9237–9246 (1999). doi:10.1021/jp990821u.
J. G. Riess, and M. P. Krafft. Advanced fluorocarbon-based systems for oxygen and drug delivery, and diagnosis. Artif. Cells Blood Substit. Immobil. Biotechnol. 25:43–52 (1997).
A. Hrabalek, P. Dolezal, O. Farsa, Z. Sklubalova, and J. Kunes. Esters of 6-dimethylaminohexanoic acid as skin penetration enhancers. Pharmazie. 55:759–761 (2000).
K. Vavrova, K. Lorencova, J. Klimentova, J. Novotny, A. N. Holy, and A. Hrabalek. Transdermal and dermal delivery of adefovir: effects of pH and permeation enhancers. Eur. J. Pharm. Biopharm. 69:597–604 (2008). doi:10.1016/j.ejpb.2007.12.005.
K. Vavrová, K. Lorencová, J. Novotný, A. Holý, and A. Hrabálek. Permeation enhancer dodecyl 6-(dimethylamino)hexanoate increases transdermal and topical delivery of adefovir; influence of pH, ion-pairing and skin species. Eur. J. Pharm. Biopharm. 70:901–907 (2008), doi:10.1016/j.ejpb.2008.07.002
A. Hrabalek, P. Dolezal, K. Vavrova, J. Zbytovska, T. Holas, J. Klimentova, and J. Novotny. Synthesis and enhancing effect of transkarbam 12 on the transdermal delivery of theophylline, clotrimazole, flobufen, and griseofulvin. Pharm. Res. 23:912–919 (2006). doi:10.1007/s11095-006-9782-y.
A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff, and R. D. Shah. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J. Org. Chem. 61:3849–3862 (1996). doi:10.1021/jo960057x.
C. Herkenne, A. Naik, Y. N. Kalia, J. Hadgraft, and R. H. Guy. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 23:1850–1856 (2006). doi:10.1007/s11095-006-9011-8.
U. Jacobi, M. Kaiser, R. Toll, S. Mangelsdorf, H. Audring, N. Otberg, W. Sterry, and J. Lademann. Porcine ear skin: an in vitro model for human skin. Skin Res. Technol. 13:19–24 (2007). doi:10.1111/j.1600-0846.2006.00179.x.
A. Williams. Alternative membranes for in-vitro studies. Transdermal and Topical Drug Delivery: From Theory to Clinical Practice, Pharmaceutical Press, London, 2003, pp. 54–58
K. Vavrova, K. Lorencova, J. Klimentova, J. Novotny, and A. Hrabalek. HPLC method for determination of in vitro delivery through and into porcine skin of adefovir (PMEA). J. Chrom. B. 853:198–203 (2007). doi:10.1016/j.jchromb.2007.03.012.
W. J. Fasano, S. C. Carpenter, S. A. Gannon, T. A. Snow, J. C. Stadler, G. L. Kennedy, R. C. Buck, S. H. Korzeniowski, P. M. Hinderliter, and R. A. Kemper. Absorption, distribution, metabolism, and elimination of 8–2 fluorotelomer alcohol in the rat. Toxicol. Sci. 91:341–355 (2006). doi:10.1093/toxsci/kfj160.
R. Fraginals, M. Schaeffer, J. L. Stampf, and C. Benezra. Perfluorinated analogues of poison ivy allergens. Synthesis and skin tolerogenic activity in mice. J. Med. Chem. 34:1024–1027 (1991). doi:10.1021/jm00107a022.
B. J. Aungst. Structure/effect studies of fatty acid isomers as skin penetration enhancers and skin irritants. Pharm. Res. 6:244–247 (1989). doi:10.1023/A:1015921702258.
J. Klimentova, P. Kosak, K. Vavrova, T. Holas, and A. Hrabalek. Influence of terminal branching on the transdermal permeation-enhancing activity in fatty alcohols and acids. Bioorg. Med. Chem. 14:7681–7687 (2006). doi:10.1016/j.bmc.2006.08.013.
J. Klimentova, P. Kosak, K. Vavrova, T. Holas, J. Novotny, and A. Hrabalek. Transkarbams with terminal branching as transdermal permeation enhancers. Bioorg. Med. Chem. Lett. 18:1712–1715 (2008). doi:10.1016/j.bmcl.2008.01.040.
D. Chantasart, S. K. Li, N. He, K. S. Warner, S. Prakongpan, and W. I. Higuchi. Mechanistic studies of branched-chain alkanols as skin permeation enhancers. J. Pharm. Sci. 93:762–779 (2004). doi:10.1002/jps.10550.
A. Hrabalek, K. Vavrova, P. Dolezal, and M. Machacek. Esters of 6-aminohexanoic acid as skin permeation enhancers: The effect of branching in the alkanol moiety. J. Pharm. Sci. 94:1494–1499 (2005). doi:10.1002/jps.20376.
J. J. Prusakiewicz, C. Ackermann, and R. Voorman. Comparison of skin esterase activities from different species. Pharm. Res. 23:1517–1524 (2006). doi:10.1007/s11095-006-0273-y.
W. Montagna. Histology and cytochemistry of human skin. IX. The distribution of non-specific esterases. J. Biophys. Biochem. Cytol. 1:13–16 (1955).
D. J. Davies, R. J. Ward, and J. R. Heylings. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. In Vitro. 18:351–358 (2004). doi:10.1016/j.tiv.2003.10.004.
A. Holy, J. Gunter, H. Dvorakova, M. Masojidkova, G. Andrei, R. Snoeck, J. Balzarini, and E. De Clercq. Structure-antiviral activity relationship in the series of pyrimidine and purine N-[2-(2-phosphonomethoxy)ethyl] nucleotide analogues. 1. Derivatives substituted at the carbon atoms of the base. J. Med. Chem. 42:2064–2086 (1999). doi:10.1021/jm9811256.
V. Kopecky Jr., P. Mojzes, J. V. Burda, and L. Dostal. Raman spectroscopy study of acid-base and structural properties of 9-[2-(phosphonomethoxy)ethyl]adenine in aqueous solutions. Biopolymers. 67:285–288 (2002). doi:10.1002/bip.10111.
Acknowledgements
This work was supported by the Centre for New Antivirals and Antineoplastics (1M0508), the Ministry of Education of the Czech Republic (MSM0021620822) and the Grant Agency of the Charles University (286/2006/B-CH/FaF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novotný, J., Kovaříková, P., Novotný, M. et al. Dimethylamino Acid Esters as Biodegradable and Reversible Transdermal Permeation Enhancers: Effects of Linking Chain Length, Chirality and Polyfluorination. Pharm Res 26, 811–821 (2009). https://doi.org/10.1007/s11095-008-9780-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9780-3