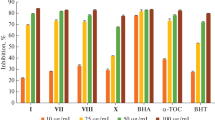
In this work, a series of seven bis-histamine Schiff bases (H1–H4) and bis-spinaceamine substituted derivatives (SPH1, SPH2, and SPH4) were successfully re-synthesized to evaluate their antioxidant properties by several bioanalytical methods such as DPPH free radical scavenging assay, ABTS radical cation decolorization assay, metal chelating and CUPRAC methods. On the other hand, these compounds were also investigated as inhibitors of acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and DNAcleavage. The results revealed that all compounds showed, in general, moderate DPPH and ABTS radical scavenging activities, and low metal chelating and CUPRAC activities. Specifically, compound SPH4 showed good DPPH radical scavenging activity with IC50 value of 59.59 μM, which is better than the BHA and BHT standard values. The best AChE and BChE inhibition results among the tested series were also obtained for compound SPH4 with % inhibition values of 84.51 and 75.89, respectively. Taken together, compound SPH4 might be an interesting lead compound to discover more potent agents against these enzymes.




Similar content being viewed by others
References
N. Lolak, M. Boga, M. Tuneg, et al., J. Enzyme Inhib. Med. Chem., 35(1), 424 – 431 (2020).
I. Zueva, J. Dias, S. Lushchekina, et al., Neuropharmacology, 155, 131 – 141 (2019).
P. Taslimi, M. Işık, F. Türkan, et al., J. Biomol. Struct. Dyn., (2020). https: //doi.org/https://doi.org/10.1080/07391102.2020.1790422.
N. Lolak, S. Akocak, C. Turkeş, et al., Bioorg. Chem., 100, 103897 (2020).
D. O. Ozgun, H. I. Gul, C. Yamali, et al., Bioorg. Chem., 84, 511 – 517 (2019).
S. V. Lushchekina and P. Masson, Neuropharmacology, 177, 108236 (2020).
S. Akocak, N. Lolak, A. Nocentini, et al., Bioorg. Med. Chem., 25, 3093 – 3097 (2017).
S. Akocak, M. R. Alam, A. M. Shabana, et al., J. Med. Chem., 59(10), 5077 – 5088 (2016).
M. Abellán-Flos, M. Tanç, C. T. Supuran, et al., J. Enzyme Inhib. Med. Chem., 31(6), 946 – 52 (2016).
M. Abellán-Flos, M. Tanç, C. T. Supuran, and S. P. Vincent, Org. Biomol. Chem., 13(27), 7445 – 7451 (2015).
B. Draghici, D. Vullo, S. Akocak, et al., Chem. Commun (Camb.), 50(45), 5980 – 5983 (2014).
S. Akocak, N. Lolak, S. Bua, et al., J. Enzyme Inhib. Med. Chem., 34(1), 1193 – 1198 (2019).
M. S. Blois, Nature, 181, 1199 – 1200 (1958)
S. Akocak, N. Lolak, M. Tuneg, and M. Boga, J. Turk. Chem. Soc. Sec. A: Chem., 6(2), 157 – 164 (2019).
N. Lolak and S. Akocak, Cumhuriyet Sci. J., 41(2), 413 – 418 (2020).
R. Re, N. Pellegrini, A. Proteggente, et al., Free Radic. Biol. Med., 26(9–10), 1231 – 1237 (1999).
S. Akocak, M. Boga, N. Lolak, et al., J. Turk. Chem. Soc. Sec. A: Chem., 6(1), 63 – 70 (2019).
M. Oguz, E. Kalay, S. Akocak, et al., J. Enzyme Inhib. Med. Chem., 35(1), 1215 – 1223 (2020).
T. C. Dinis, V. M. Madeira and L. M. Almeida, Arch. Biochem. Biophys., 315(1), 161 – 170 (1994).
N. Lolak, M. Tuneg, A. Doğan, et al., Bioorg. Med. Chem. Rep., 3(2), 22 – 31 (2020).
G. L. Ellman, K. D. Courtney, V. Andres, and R. M. Featherstone, Biochem. Pharmacol., 7, 88 – 95 (1961).
O. Gerlits, K. Y. Ho, X. Cheng, et al., Chem. Biol. Interact., 309, 108698 (2019).
A. Meden, D. Knez, M. Jukiè, et al., Chem. Commun. (Camb.), 55(26), 3765 – 3768 (2019).
M. Iþýk, S. Akocak, N. Lolak, et al., Archiv der Pharmazie, 353(9), 2000102 (2020).
M. Durgun, C. Türkeş, M. Işık, et al., J. Enzyme Inhib. Med. Chem., 35(1), 950 – 962 (2020).
M. Işık, Y. Demir, M. Durgun, et al., Chem. Papers, 74, 1395 – 1405 (2020).
M. Bingul, S. Ercan and M. Boga, J. Mol. Struct., 1213, 128202 (2020).
P. R. Reddy and P. Manjula, Chem. Biodiver., 4(3), 468 – 480 (2007).
S. I. Kirin, C. M. Happel, S. Hrubanova, et al., Dalton Trans., 21(8), 1201 – 1207 (2004).
S. 30. S. Massoud, F. R. Louka, W. Xu, et al., Eur. J. Inorg. Chem., 2011(23), 3469 – 3479 (2011).
U. Chaveerach, A. Meenongwa, Y. Trongpanich, et al., Polyhedron, 29(2), 731 – 738 (2010).
S. S. Tonde, A. S. Kumbhar, S. B. Padhye, and R. J. Butcher; J. Inorg. Biochem., 100(1), 51 – 57 (2006).
Acknowledgements
This work was partially supported by The Scientific and Technological Research Council of Turkey (TUBITAK), Research Fund Project No. 215Z484.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 1, pp. 10 – 10, January, 2022.
Rights and permissions
About this article
Cite this article
Lolak, N., Boga, M., Sonmez, G.D. et al. In Silico Studies and DNA Cleavage, Antioxidant, Acetylcholinesterase, and Butyrylcholinesterase Activity Evaluation of Bis-Histamine Schiff Bases and Bis-Spinaceamine Substituted Derivatives. Pharm Chem J 55, 1338–1344 (2022). https://doi.org/10.1007/s11094-022-02581-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02581-7