Abstract
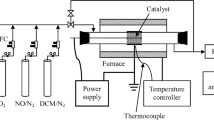
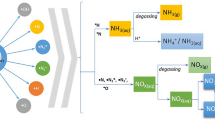
The recombination (synthesis and conversion) of nitric oxide was investigated using dielectric barrier discharge reactor at atmospheric pressure. In this work, products identification and its evolution of different gas components have been studied. In the NO/O2/N2 systems, nitric oxide (NO) can be removed via chemical oxidation and chemical reduction, and corresponding products are NO2 and N2, respectively. In the O2/N2 systems, N2O5 producing from the interaction of NO3 with NO2 was also observed. There is an optimum SED at which the highest NOx yield and best NO conversion efficiency will be achieved. In the H2O/O2/N2 systems, the formation of NO2, HNO2 and HNO3 were observed in both NF and NO conversion. The N2O molecule, as a byproduct of plasma chemical reaction, was observed in all the experiments when the H2O or O2 is presence in the simulated gas. The lowest energy cost of NO conversion is achieved at the SED of 1250 J/L.










Similar content being viewed by others
References
Hessel V, Anastasopoulou A, Wang Q, Kolb G, Lang J (2013) Catal Today 211:9–28
Penetrante BM, Hsiao MC, Merritt BT, Vogtlh GE, Wallman PH (1995) IEEE Trans Plasma Sci 23:679–689
McLarnon CR, Penetrante BM (1998) Society of automotive engineers fall fuels and lubricants meeting 1998, San Francisco, CA, pp 19–22
Kossyi AYKIA, Matveyev AA, Silakov VP (1992) Plasma Sources Sci Technol 1:207–220
Fridman A, Chirokov A, Gutsol A (2005) J Phys D Appl Phys 38:R1–R24
Teodoru S, Kusano Y, Bogaerts A (2012) Plasma Process Polym 9:652–689
McLarnon CR, Penetrante BM (1998) Society of automotive engineers fall fuels and lubricants meeting, p 982434
Patil BS, Cherkasov N, Lang J, Ibhadon AO, Hessel V, Wang Q (2016) Appl Catal B 194:123–133
Miessner H, Francke KP, Rudolph R (2002) Appl Catal B Environ 36:53–62
Tonkyn RG, Barlow SE, Hoard JW (2003) Appl Catal B 40:207–217
Atkinson R, Baulch DL, Cox RA, Hampson RF, Kerr JA, Rossi MJ, Troe J (1997) J Phys Chem Ref Data 26:1329
Fridman A (2012) Plasma chemistry. Cambridge University Press, New York
Penetrante BM, Brusasco RM, Merritt BT, Pitz WJ, Vogtlin GE, Kung MC, Kung HH, Wan CZ, Voss KE (1998) Society of automotive engineers fall fuels and lubricants meeting, p 982508
Fujii YAT, Yoshioka N, Rea M (2001) J Electrostat 51–52:8–14
Zhu A-M, Sun Q, Niu J-H, Xu Y, Song Z-M (2005) Plasma Chem Plasma Process 25:371–386
Jõgi I, Levoll E, Raud J (2016) Chem Eng J 301:149–157
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds. John Wiley & Sons, Inc., Hoboken, New Jersey
Hadjiivanov KI (2000) Catal Rev 42:71–144
Grundmann CTS (2009) Int J Heat Fluid Flow 30:394–402
Borcia G, Anderson CA, Brown NM (2003) Plasma Sources Sci Technol 12:335–344
Kantcheva M, Cayirtepe I (2006) J Mol Catal A Chem 247:88–98
Bibart CH, Ewing GE (1974) J Chem Phys 61:1284–1292
Cantrell CA, Davidson JA, McDaniel AH, Shetter RE, Calvert JG (1988) Chem Phys Lett 148:358–363
Guillory WA, Hunter CE (1971) J Chem Phys 54:598–603
McGraw GE, Bernitt DL, Hisatsune IC (1965) J Chem Phys 42:237
Al-Abduly A, Christensen P (2015) Plasma Sources Sci Technol 24:065006
Golde M (1988) Int J Chem Kinet 20:75–92
Wang T, Sun B-M, Xiao H-P, Wang D, Zhu X-Y, Zhong Y-F (2013) Plasma Chem Plasma Process 33:681–690
Yin S-E, Sun B-M, Gao X-D, Xiao H-P (2009) Plasma Chem Plasma Process 29:421–431
Jones LH, Badger RM, Moore GE (1951) J Chem Phys 19:1599
Eliasson B, Hirth M, Kogelschatz U (1987) J Phys D Appl Phys 20:1421–1437
Zhao G-B, Hu X, Argyle MD, Radosz M (2004) Ind Eng Chem Res 43:5077–5088
Higashi SUM, Suzuki N, Fujii K, Trans IEEE (1992) Plasma Sci 20:1–12
NIST chemical kinetics database. http://kinetics.nist.gov/kinetics/index.jsp
Green JHD (2001) Plasma Chem Plasma Process 21:459–481
Acknowledgements
This study was primarily supported by National Key R&D Program of China (2017YFC0210303-01). This work was also partly supported by National Natural Science Foundation of China (21677010, U1660109).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, X., Wang, J., Yi, H. et al. Nitrogen Fixation and NO Conversion using Dielectric Barrier Discharge Reactor: Identification and Evolution of Products. Plasma Chem Plasma Process 38, 485–501 (2018). https://doi.org/10.1007/s11090-018-9876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-018-9876-4